Introduction
Understanding how wildlife adapts to
seasonal changes is crucial for predicting their responses to environmental
fluctuations and for developing effective conservation strategies (Fuller et al. 2010; Beever et al.
2017). This study focuses on the mountain hare (Lepus
timidus), a species that exhibits a remarkable adaptation to its
environment through seasonal fur colour changes (Flux 1970; Stoner et al. 2003). The transition from brown summer fur to white winter fur is an
essential survival strategy that provides camouflage against predators in snowy
landscapes (Zimova et al.
2020a). However, the timing of this fur change can
be influenced by various environmental factors, and mismatches between fur
colour and the surrounding environment can increase predation risk and impact
population dynamics (Mills et
al. 2013; Atmeh et al. 2018).
The timing of fur change in the mountain
hare is primarily driven by environmental cues that signal the approach of
winter (Stokes et al. 2023). Elevation and latitude are two critical factors that influence
the onset and duration of winter conditions, and consequently, the timing of
fur change. At higher elevations, temperatures drop earlier in the season, and
snow cover persists longer, necessitating an earlier fur change in the autumn,
but later fur change in the spring. Similarly, higher latitudes experience more
pronounced seasonal changes, which can also affect the timing of fur colour
transition.
Temperature is a key determinant of the
timing of fur change (Jackes
and Watson 1975; Stokes et al. 2023). As temperatures
drop, physiological changes in the mountain hare trigger the growth of white
fur, which is better suited to snowy environments (Zimova et al. 2018). Photoperiod,
or daylength, is a key parameter influencing the timing of seasonal fur changes
in mountain hares, either alone or alongside other variables like snow cover (Watson 1963; Flux 1970; Hofman 2004).
Shortening days signal the approach of winter, triggering hormonal changes that
start the moulting process (Ferreira et al. 2020). Additionally,
snow cover serves as a direct environmental cue, with its presence and duration
affecting the timing and extent of fur color change, ensuring effective
camouflage throughout winter (Zimova et al. 2016).
In addition to these primary factors, other
environmental variables can play a role in the timing of fur change. Vegetation
type and cover can influence microclimates, affecting local temperatures and
snow retention (Peltier et
al. 2023). Wind patterns and precipitation can also
impact snow cover dynamics, further influencing the hare’s camouflage needs (Jones et al. 2020). Moreover, the interaction between these factors can create
complex environmental conditions that affect the timing of fur change in
different ways (Zimova et al.
2016).
Understanding the interplay of these
factors is crucial, especially in the context of climate change. Rising global
temperatures and altered precipitation patterns are leading to changes in snow
cover dynamics (Brown and
Mote 2009). In many regions, snow arrives later and
melts earlier, potentially disrupting the synchronization between fur change
and snow cover (Semenchuk
et al. 2013). This asynchrony can leave hares
vulnerable to predation during periods when their white fur stands out against
a snowless background (Zimova
et al. 2014). Such changes not only impact
individual hares but can also have broader implications for population dynamics
and ecosystem health (Mills et
al. 2013).
In addition, it is important to consider
the genetic factors that may influence the timing of fur change. Genetic
variation within and between populations can result in different adaptive
strategies to local environmental conditions (Boursot et al. 1993; Feder et al. 2012). This genetic diversity can be a crucial element in the resilience
of species to rapidly changing climates.
Recent advances in research tools, such as
camera traps, have significantly enhanced our ability to monitor wildlife
behaviour and ecological processes across diverse habitats (O'Connell et al. 2011). In the context of this study, camera traps represent a valuable
tool for documenting the timing of winter fur change in mountain hares (Bison et al. 2024). By providing non-invasive, time-stamped visual records, camera
traps enable researchers to track seasonal changes in fur colouration in
relation to environmental variables, offering critical insights into the
spatiotemporal dynamics of this adaptation. As this study is part of a special
issue on camera traps, it highlights the potential of this technology to
support long-term monitoring efforts and inform conservation strategies.
While the seasonal fur colour change of
mountain hares has been widely studied, the influence of elevation and latitude
on this adaptation remains underexplored. Previous research has largely focused
on factors such as snow cover or photoperiod, often without accounting for
their geographic variation or the potential interplay between these variables.
This study contributes to filling this gap by examining how fur colour
transitions are influenced by snow presence, latitude, and altitude across
different regions, using detailed records obtained through camera traps. By
integrating these environmental variables, this work aims to provide insights
into the drivers of phenological adaptations in mountain hares.
In this context, the primary objectives of
this study are i) to analyse the timing of winter fur change in the mountain
hare across different elevations and latitudes, and ii) to assess the
relationship between fur change timing and various environmental factors, including
snow presence, latitude, and altitude. By gathering and analysing data from
diverse habitats, this study aims to provide a comprehensive understanding of
the factors driving fur change. Through detailed examination of the mountain
hare's adaptive strategies, this research seeks to contribute valuable insights
into the broader field of phenology and species adaptation. The findings will
be essential for informing conservation efforts aimed at preserving mountain
hare populations and maintaining ecosystem balance in the face of ongoing and
future climate change.
Material and methods
Study area
The study was conducted using images from
camera traps that were deployed by the University of Gävle in two different
areas of the county of Gävleborg in Sweden (Fig. 1), to monitor ungulate
populations. The Ockelbo region in central Sweden is characterised by extensive
forests that form part of the dominant landscape. The forests in this area are
mainly composed of coniferous trees, such as Scots pine (Pinus silvestris)
and Norway spruce (Picea abies), although some areas of mixed forest
with deciduous trees such as birch (Betula spp.) can also be found. The
second area, Kårböle, is situated in a more mountainous and rugged region,
giving it a more uneven forested landscape with hills covered by dense forests.
These forests tend to be more continuous and less fragmented, providing a more
isolated habitat for wildlife. There was a
widespread forest fire in this area in 2018. The size of the Ockelbo study area
was 24 650 hectares, and the size of the Kårböle study area was
12 750 hectares, with camera traps deployed in each area at a density of
one camera per 850 hectares. The total area covered by the cameras was 9 106 m2
in Ockelbo and 4710 m2 in Kårböle.
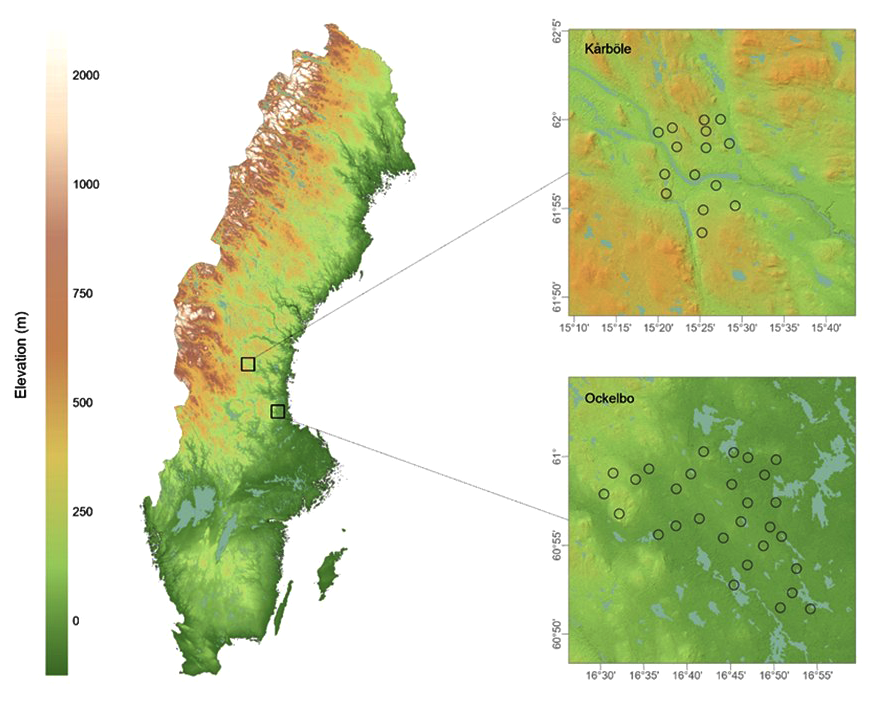
Figure
1. Map of Sweden (left) with inserts showing the
two study areas (right) and the location of the cameras (black circles), as
well as the latitude and altitude of each camera. Elevation data was obtained
from Markhöjdmodell Nedladdning, grid 1+ ©Lantmäteriet (2021).
Figura 1. Mapa de Suecia (izquierda) con las dos áreas de estudio
(derecha) que muestra la ubicación de las cámaras (los círculos negros), así
como la latitud y altitud de cada cámara. Los datos de elevación se obtuvieron
de Markhöjdmodell Nedladdning, cuadrícula 1+ ©Lantmäteriet (2021).
Sampling design
Images of mountain hares were collected
from 44 Reconyx HyperFire 2 HF2X cameras (29 in Ockelbo and 15 in Kårböle) between June 2020 and October 2022, with the earliest image
captured on 20 June 2020 and the latest on 31 October 2022, resulting in 1948
observations of mountain hares from the two areas. (25 cameras in Ockelbo, with
an efficiency of 0.057 observation/day and 15 cameras in Kårböle with an efficiency of 0.089 observation/day). The camera
traps effort was 25 504 days in Ockelbo and 11 213 in Kårböle.
The cameras were placed at salt lick stones
near a water source. The cameras were mounted facing north approximately 1.5
meters above ground (± 20 cm) at an average distance of 10 meters from the pole
holding the salt lick stone. The capturing angle of the HF2X is 40º and the maximum reliable detection range of the PIR sensor is 30
meters, providing a total theoretical capturing area of 314 m2/camera
(1/9th of the area of a circle with the radius of 30 meters). ** The
range of altitude for the camera trap locations was 63-260 m above sea level in
Ockelbo, and 228-353 m above sea level in Kårböle.
To trigger the camera, two criteria have to
be fulfilled. The first is that the object must have a temperature different
from the surrounding environment. The second is that the object with a
temperature difference has to move horizontally within the active zone of the
camera, approximately 1/8th of the distance across the field of
view.
All cameras were configured to take a set
of 4 pictures (one per second) when triggered, followed by a 1-hour delay
before they could be triggered again.
When an individual animal appeared in
multiple pictures, consecutive images were considered a single observation
unless more than an hour had elapsed between captures. Observations where the
images did not provide sufficient detail to assess fur colour were discarded,
resulting in 1,656 observations for the study.
For each mountain hare observation, we made
a record of fur colour by estimating the proportion of the animal’s coat that
was white (excluding the legs and belly), and classifying moulting stage into
three categories, modified from Zimova et al. (2020b) and Stokes et al. (2023). Hares with ≥90% white fur were classified as ‘white’, hares with
≤10% white fur were classified as ‘brown’, and all other hares were classified
as ‘moulting’ (Figure A1 of the Annex).
Environmental cues
We classified ground snow cover into three
categories, based on the amount of snow visible in the images: ‘Full snow
cover’, ‘partial snow cover’, and ‘no snow’. 'Full snow cover' if there were no
patches of visible lower vegetation, 'no snow' if there was no snow whatsoever,
and 'partial snow cover' for everything in between. Altitude and latitude were
extracted based on camera trap positions, with altitude, specifically, obtained
from a digital terrain model (DTM) with a resolution of 1x1 meter (Lantmäteriet 2021), using ArcGIS Pro 3.2.2 (Esri 2024).
Statistical analysis
Prior to building the models, collinear
variables with Variation Inflation Factors (VIF) > 3 were removed (Zuur et al. 2009; Zuur et al. 2007), which were study area, temperature and day of the year
(photoperiod) due to their strong collinearity with snow cover, a variable that
we prioritized as the most direct and ecologically relevant predictor for fur
colour change.
The statistical analysis was performed to
evaluate the effects of environmental factors, including snow presence,
altitude, and latitude, on the fur colour of the studied species. The analysis
also considered the potential interaction between altitude and latitude. A
multinomial generalized linear model (GLM) was fitted to the data with fur
colour as the response variable (three levels; white, brown and moulting),
implemented via the “multinom” function from the “nnet” package in R (Venables and Ripley 2002).
The predictors included in the model were
snow presence (a categorical variable with three levels: no snow, partial and
full snow cover), altitude (a continuous variable), and latitude (a continuous
variable). The interaction between altitude and latitude was also included to
assess whether the effect of latitude on fur colour varied with changes in
altitude. Given the potential for unequal group sizes and unbalanced data, Type
III sum of squares was employed in the analysis. This method is appropriate when
the design is not fully balanced, allowing for the assessment of each
predictor's effect while accounting for the presence of other variables in the
model. The significance of the main effects and the interaction term was tested
using Type III ANOVA followed by a post-hoc Tukey’s HSD test to identify
significant pairwise differences between seasons, implemented via the “Anova”
function from the “car” package in R (Fox and Weisberg 2019). The likelihood
ratio chi-square test was used to determine the statistical significance of
each factor. The model's fit was assessed, and p-values were reported for each
predictor to determine its influence on fur colour. A significance level of
α=0.05 was used for all statistical tests. Results were considered
statistically significant if the p-value was less than 0.05. The statistical
analysis was conducted using R software, version [R version 4.4.0 (2024-04-24)]
(R Core Team 2024). We evaluated spatial and temporal autocorrelation in the data to
ensure the robustness of the model's inferences. Moran’s I was used to test for
spatial autocorrelation in the residuals, and no significant patterns were
detected. Similarly, temporal autocorrelation was assessed using the
autocorrelation function (ACF), which showed no significant dependency over
time. These findings suggest that spatial and temporal autocorrelation are
unlikely to have influenced the results.
Results
Descriptive results
In total, we
obtained 1656 records of mountain hares (849 in Ockelbo and 807 in Kårböle),
with 699 observations of white hares, 720 of brown hares, and 327 of moulting
hares. The frequency of colour distribution in the two study areas was similar
(chi-square= 1.71, p=0.42) (Fig.
2).
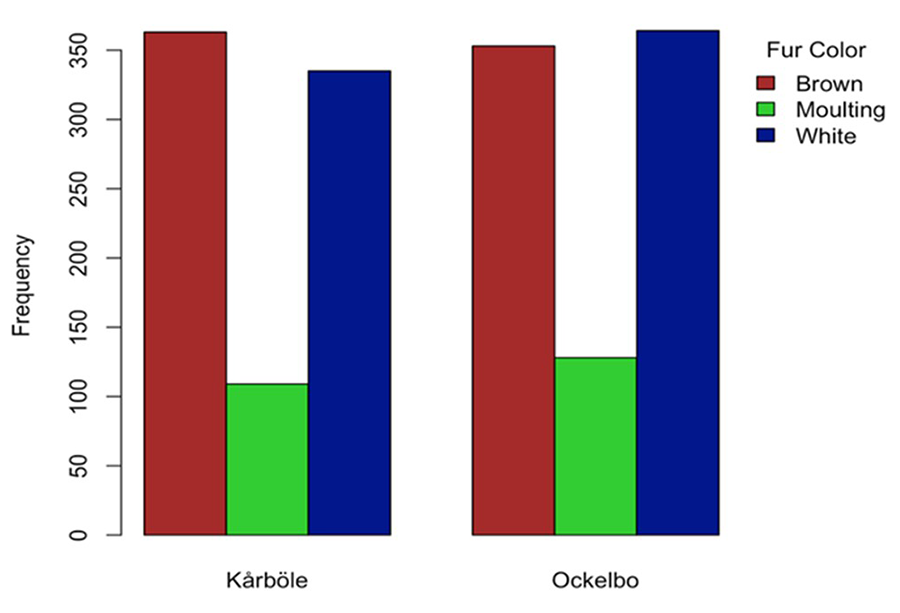
Figure
2. Colour frequency according to the study area
(not significant p>0.05).
Figura 2. Frecuencia de
color según zona de estudio (no significativo p>0.05).
Factors that determine colour
change
The analysis revealed a highly significant
effect of snow on fur colour. This suggests that snow presence plays a critical
role in determining the fur colour (Figure A2 of the Annex),
likely as an adaptive response to camouflage in snowy environments (Fig. 3). Latitude
also showed a statistically significant effect on fur colour (Table 1). This
finding indicates that fur colour varies with latitude, possibly reflecting
environmental or climatic gradients that influence selective pressures on fur
pigmentation (Fig. 4).
Table 1. Chi square and p value of
the multinomial model.
Tabla 1. Chi cuadrado y
valor p del modelo multinomial.
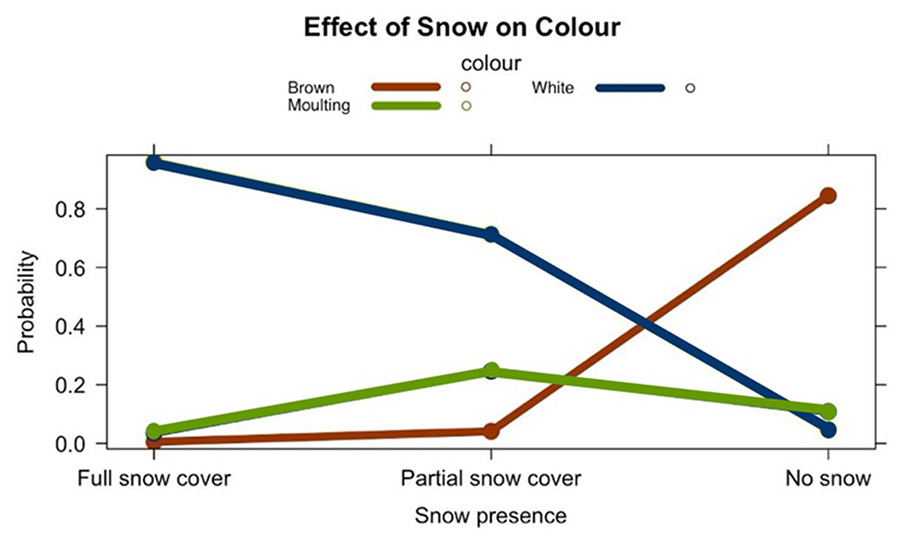
Figure
3. Probability of frequency of each colour (0 to 1)
according to snow cover.
Figura 3. Probabilidad de frecuencia de cada color
(0 a 1) según la cobertura de nieve.
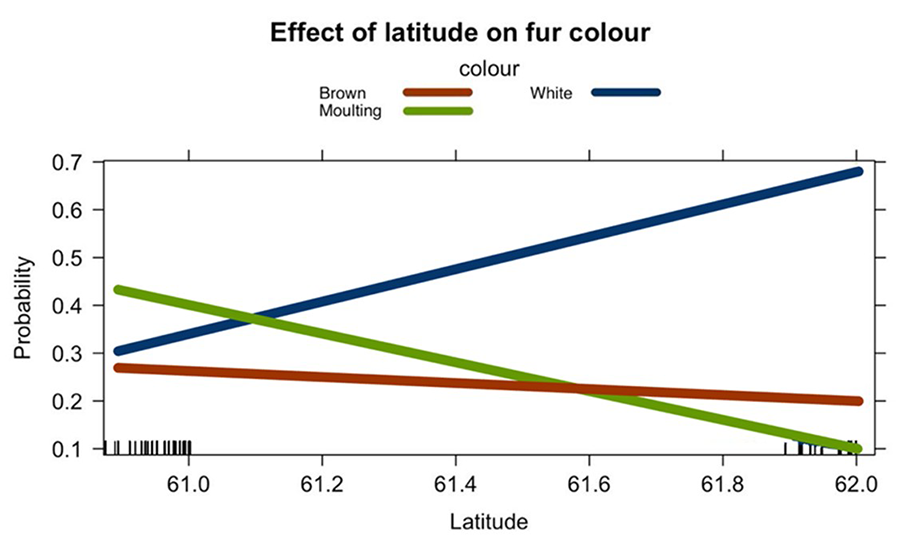
Figure
4. Relationship between the probability of each
colour and latitude.
Figura 4. Relación entre la probabilidad de cada
color y la latitud.
In contrast, altitude did not have a
significant main effect on fur colour. Similarly, the interaction between
altitude and latitude was not significant, suggesting that the effects of
latitude and altitude on fur colour are independent and do not interact in a
meaningful way. However, we found that the altitude at which hares are found
depends on the season, being at a higher altitude in summer and autumn,
followed by a lower altitude in spring and finally the lowest altitude in
winter (Fig. 5). This seasonal altitudinal pattern coincides with the variation in
colour of hares according to seasonality (Fig. 6).
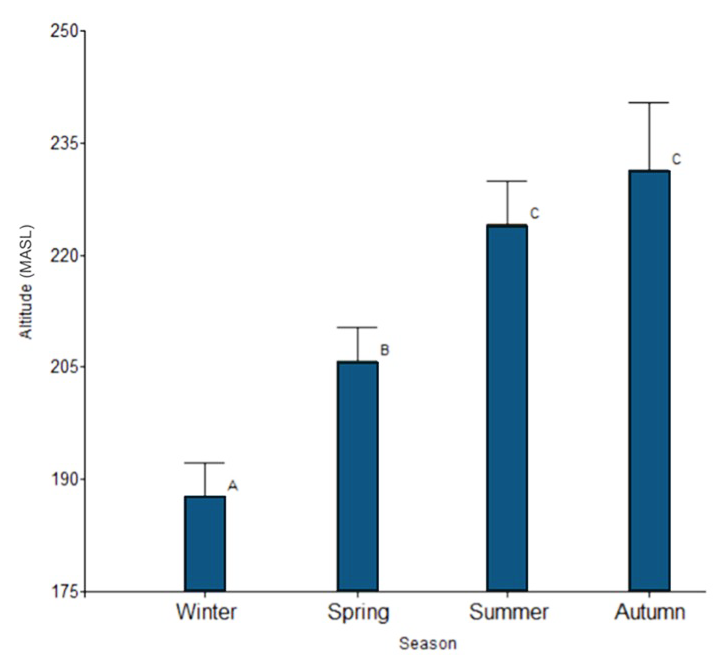
Figure
5. Changes in altitude according to season.
Different letters indicate significant differences (p<0.05) based on a
post-hoc Tukey’s HSD test following a one-way ANOVA.
Figura 5. Cambios en la
altitud según la estación. Letras diferentes indican diferencias significativas
(p<0,05) según un análisis post-hoc Tukey’s HSD realizado después de un
ANOVA de una vía.
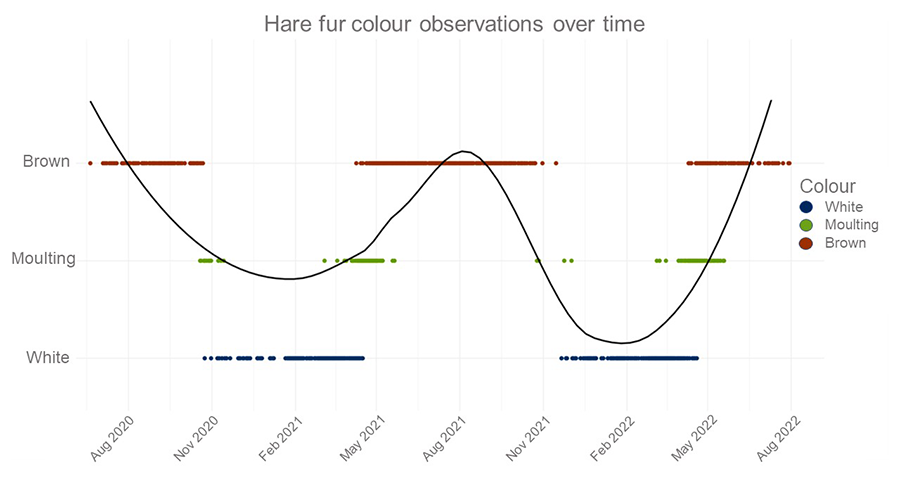
Figure
6. Changes in the colour of the hare's fur
according to seasonality. The black line represents the seasonal trend in fur
colour, based on fitted values from the statistical model.
Figura 6. Cambios en el color del pelaje de la
liebre según la estacionalidad. La línea negra representa la tendencia
estacional en el color del pelaje, basada en los valores ajustados del modelo
estadístico.
Discussion
This study provides a comprehensive
understanding of how key environmental factors —snow cover, latitude, and
altitude—drive fur colour changes in the mountain hare (Lepus timidus) –
a critical adaptation for survival in environments with pronounced seasonal
fluctuations (Mills et al.
2013; Zimova et al. 2014). The results reveal
the crucial importance of snow presence and latitude as determinants of fur
colour within the studied range,
while altitude did not show a significant effect. The
lack of interaction between altitude and latitude further suggests that these
factors operate independently in shaping the observed patterns of fur colour.
The statistical analysis demonstrated that
snow presence is the most influential factor in determining fur colour. This
finding is consistent with previous studies suggesting that snow acts as a
powerful environmental cue, prompting the transition to white fur for
camouflage in snowy landscapes (Mills et al. 2013; Zimova et al. 2014). The strong correlation between snow cover and fur colour
highlights the hare’s adaptation to its environment, where the ability to blend
into the snow is critical for avoiding predators (Zimova et al. 2016; Kennah 2022; Oli et al. 2023).
Latitude exhibited a statistically
significant effect on fur colour, indicating that fur colour variation is
influenced by latitudinal gradients (Stokes et al. 2023). This finding
suggests that geographic differences, as determined by latitude, play a crucial
role in shaping fur pigmentation. Latitudinal gradients often correlate with
variations in environmental or climatic conditions, such as changes in
photoperiod (Ferreira et
al. 2017), temperature, and (Zimova et al. 2014) seasonal weather patterns, which can impose different selective
pressures on fur colour (Peltier
et al. 2023).
In higher latitudes, where winter
conditions are more severe and snow cover is more persistent, animals typically
exhibit a shift to lighter fur colours to enhance camouflage and avoid
predation. Conversely, in lower latitudes, where snow cover is less frequent or
shorter in duration, the transition to a white winter coat may be less
pronounced or even absent. This variation can be attributed to differences in
photoperiod, where longer winter nights in higher latitudes may signal the need
for a seasonal colour change. Additionally, temperature fluctuations and
snowfall patterns can directly influence the timing and extent of the fur
colour transition.
In contrast,
altitude did not have a significant main effect on fur colour, nor did the
interaction between altitude and latitude. This lack of significance suggests
that the effects of latitude and altitude on fur colour operate independently
and do not interact in a meaningful way within the studied population. While
higher altitudes generally lead to earlier onset of winter conditions, this
factor alone does not appear to drive the fur colour change as strongly as snow
presence or latitude. Flux (1970) found that colours showed no definite correlation
with altitude. However, Stokes et al. (2023) found that mountain hare moult timing is strongly
correlated with altitude and latitude with hares that live at higher latitudes
and altitudes keeping their winter white coats for longer than their
conspecifics that inhabit lower latitudes and altitudes. However, these authors
had a much broader altitude range (0–841 m). The
altitudinal range in this study (63–353 m above sea level) represents a subset
of the broader altitudinal distribution of mountain hares in Sweden, which
extends to the upper reaches of the Caledonian Mountains. This limited range
may partially explain the lack of a significant effect of altitude on fur
colour change observed in our results. In higher altitudes, where snow cover
tends to persist longer and winter conditions are harsher, the relationship
between altitude and fur colour may become more pronounced. Future studies
incorporating a broader altitudinal gradient could provide additional insights
into how this factor influences seasonal adaptations in mountain hares.
Seasonal
altitudinal movements may be responsible for the lack of differences. At the
microniche scale (as in this study), the availability of food and shelter
drives the altitudinal movement of hares according to the seasons (Bison et al.
2024). During the
winter, hares may descend to lower areas where food is more accessible (Rehnus
and Bollmann 2020).
These lower regions tend to have less snow accumulation or offer more shelter
and food sources, such as branches and bushes that protrude from the snow. By
contrast, during the summer and autumn months, when hares change to their brown
coat, they may migrate to higher altitudes where fresh and abundant vegetation
is available. Higher altitudes provide new grasses and herbaceous plants that
hares prefer during the growing season (Rehnus and Bollmann 2020). This pattern of seasonal movement,
related to foraging, is common in many species inhabiting mountainous regions.
Additionally, shifting locations with the seasons not only maximizes food
availability but may also help hares reduce predator exposure by taking
advantage of seasonal camouflage provided by their fur.
Understanding the interplay of these
environmental factors is particularly important in the context of climate
change. As global temperatures rise and snow cover patterns shift, the
synchronization between fur colour change and the environment may become
disrupted. Such mismatches could increase the vulnerability of hares to
predation, potentially leading to declines in population numbers. Conservation
strategies should therefore consider the potential impacts of changing snow
cover dynamics and aim to preserve habitats that support the natural camouflage
and survival of mountain hares.
Conclusion
In conclusion, our results show that camera
traps provide detailed information on mountain hare ecology and confirm the
importance of working at different scales. Overall, this study emphasizes the
dominant role of snow cover and latitude in driving fur colour change in
mountain hares, while highlighting the relatively minor role of altitude, since
it is at a microniche scale, it is related to seasonal altitudinal movements.
These findings contribute to a broader understanding of phenological adaptations
in wildlife and underscore the importance of monitoring environmental changes
that could threaten these adaptive traits.
Authors' contribution
Anna Göransson:
Data curation, Visualization, Methodology, Writing – Review and editing. Davide
Carniato: Research, formal analysis, Writing – initial draft. Lars Hillstrom: Funding
acquisition, Resources, Writing – Review and editing. Petter
Hillborg: Data curation, Methodology. Marcus Larsson: Resources, Writing – Review
and editing. Antonio J. Carpio:
Conceptualization, Supervision, Writing – Review and editing.
Data availability
Data of this study is available on the
repository https://doi.org/10.5281/zenodo.14912867
Financing, required permits, potential
conflicts of interest and acknowledgments
We would like to thank the respective
forest owners of the Ockelbo Management Unit, Gammelvallen, and Ängra
Management Units. The fieldwork was made possible thanks to grants from the
Swedish Forest Agency.
The authors declare that they have no
conflicts of interest.
References
Atmeh, K., Andruszkiewicz, A., Zub, K. 2018. Climate change is affecting
mortality of weasels due to camouflage mismatch. Scientific Reports 8(1),
7648. https://doi.org/10.1038/s41598-018-26057-5
Beever, E.A., Hall, L.E., Varner, J., Loosen, A.E., Dunham, J. B., Gahl,
M.K., et al. 2017. Behavioral flexibility as a mechanism for coping with
climate change. Frontiers in Ecology and the Environment 15(6),
299-308. https://doi.org/10.1002/fee.1502
Bison, M., Yoccoz, N.G., Carlson, B.Z., Bayle, A., Delestrade, A. 2024.
Camera traps reveal seasonal variation in activity and occupancy of the Alpine
Mountain hare Lepus timidus varronis. Wildlife Biology e01186. https://doi.org/10.1002/wlb3.01186
Boursot, P., Auffray, J.C., Britton-Davidian, J., Bonhomme, F. 1993. The
evolution of house mice. Annual Review of Ecology and Systematics
24,119-152. https://doi.org/10.1146/annurev.es.24.110193.001003
Brown, R.D.,
Mote, P.W. 2009. The response of Northern
Hemisphere snow cover to a changing climate. Journal of Climate 22(8),
2124-2145. https://doi.org/10.1175/2008JCLI2665.1
Esri. 2024. ArcGIS Pro (Version 3.2.2) [Computer software]. https://www.esri.com/
Feder, J.L., Egan, S.P., Nosil, P. 2012. The genomics of
speciation-with-gene-flow. Trends in Genetics 28(7), 342-350. https://doi.org/10.1016/j.tig.2012.03.009
Ferreira, M.S., Alves, P.C., Callahan, C.M., Marques, J.P., Mills, L.S.,
Good, J.M., Melo‐Ferreira, J. 2017. The transcriptional landscape of seasonal coat
colour moult in the snowshoe hare. Molecular
Ecology 26(16), 4173-4185. https://doi.org/10.1111/mec.14177
Ferreira, M.S., Alves, P.C., Callahan, C.M., Giska, I., Farelo, L., Jenny, H.,
et al. 2020. Transcriptomic regulation of seasonal coat
color change in hares. Ecology and Evolution 10(3), 1180-1192.
https://doi.org/10.1002/ece3.5956
Fuller, A., Dawson, T., Helmuth, B., Hetem, R.S., Mitchell, D., Maloney,
S.K. 2010. Physiological mechanisms in coping with climate change. Physiological
and Biochemical Zoology 83(5), 713-720. https://doi.org/10.1086/652242
Flux, J.E. 1970. Colour change of Mountain hares (Lepus timidus
scoticus) in north‐east Scotland. Journal of Zoology 162(3), 345-358. https://doi.org/10.1111/j.1469-7998.1970.tb01270.x
Fox, J,
Weisberg. S. 2019. An R Companion to Applied
Regression, Third edition. Sage, Thousand Oaks CA. https://www.john-fox.ca/Companion/
Hofman, M.A. 2004. The brain's calendar: Neural mechanisms of seasonal
timing. Biological Reviews of the Cambridge Philosophical Society 79,
61–77. https://doi.org/10.1017/S1464793103006250
Jackes,
A.D., Watson, A. 1975. Winter whitening of Scottish
Mountain hares (Lepus timidus scoficus) in relation to daylength,
temperature and snow-lie. Journal of Zoology 176, 403–409. https://doi.org/10.1111/j.1469-7998.1975.tb03211.x
Jones, M.R., Mills, L.S., Jensen, J.D., Good, J.M. 2020. Convergent
evolution of seasonal camouflage in response to reduced snow cover across the
snowshoe hare range. Evolution 74(9), 2033-2045. https://doi.org/10.1111/evo.13976
Kennah, J. 2022. An evaluation of the costs and responses of coat
colour mismatch in snowshoe hares. Masters thesis,
Memorial University of Newfoundland. https://doi.org/10.48336/MXSP-H118
Lantmäteriet 2021. Markhöjdmodell Nedladdning, grid 1+.
[GeoTIFF]. https://www.lantmateriet.se/sv/geodata/vara-produkter/produktlista/markhojdmodell-
nedladdning-grid-1/
Mills, L.S., Zimova, M., Oyler, J., Running, S., Abatzoglou, J.T.,
Lukacs, P.M. 2013. Camouflage mismatch in seasonal coat color due to decreased
snow duration. Proceedings of the National Academy of Sciences 110(18),
7360-7365. https://doi.org/10.1073/pnas.1222724110
O'Connell, A.F., Nichols, J.D., Karanth, K.U. 2011. Camera Traps in Animal
Ecology: Methods and Analyses. Springer. https://doi.org/10.1007/978-4-431-99495-4
Oli, M.K., Kenney, A.J., Boonstra, R., Boutin, S., Murray, D.L., Peers,
M.J., et al. 2023. Does coat colour influence survival? A test in a cyclic
population of snowshoe hares. Proceedings of the Royal Society B 290(1996),
20221421. https://doi.org/10.1098/rspb.2022.1421
Peltier, T.R., Shiratsuru, S., Zuckerberg, B., Romanski, M., Potvin, L.,
Edwards, A., et al. 2023. Phenotypic variation in the molt characteristics of a
seasonal coat color-changing species reveals limited resilience to climate
change. Oecologia 202(1), 69-82. https://doi.org/10.1007/s00442-023-05371-4
R Core
Team. 2024. R: A language and environment
for statistical computing, version 4.4.0
(2024-04-24). [Software].
R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Rehnus,
M., Bollmann, K. 2020. Mountain hares Lepus timidus
follow the green‐up wave in the pursuit of high‐quality
food. Wildlife Biology 2020(3), 1-5. https://doi.org/10.2981/wlb.00720
Semenchuk, P.R., Elberling, B., Cooper, E.J. 2013. Snow cover and extreme
winter warming events control flower abundance of some, but not all species in
high arctic S valbard. Ecology and Evolution 3(8), 2586-2599. https://doi.org/10.1002/ece3.648
Stokes, A.W., Hofmeester, T.R., Thorsen, N.H., Odden, J., Linnell, J.D.,
Pedersen, S. 2023. Altitude, latitude and climate zone as determinants of
mountain hare (Lepus timidus) coat colour change. Ecology and Evolution 13(10),
e10548. https://doi.org/10.1002/ece3.10548
Stoner, C.J., Bininda-Emonds, O.R., Caro, T.I.M. 2003. The adaptive
significance of coloration in lagomorphs. Biological Journal of the Linnean
Society 79(2), 309-328. https://doi.org/10.1046/j.1095-8312.2003.00190.x
Venables,
W.N. Ripley, B.D. 2002. Modern
Applied Statistics with S., Fourth Edition. Springer, New York. ISBN
0-387-95457-0. https://doi.org/10.1007/978-0-387-21706-2_14
Watson, A. 1963. The effect of climate on the colour changes of mountain
hares in Scotland. Proceedings of the Zoological Society of London 141,
823–835. https://doi.org/10.1111/j.1469-7998.1963.tb01629.x
Zimova, M., Mills, L.S., Lukacs, P.M., Mitchell, M.S. 2014. Snowshoe hares
display limited phenotypic plasticity to mismatch in seasonal camouflage. Proceedings
of the Royal Society B: Biological Sciences 281(1782), 20140029. https://doi.org/10.1098/rspb.2014.0029
Zimova, M., Mills, L.S., Nowak, J.J. 2016. High fitness costs of climate
change‐induced camouflage mismatch. Ecology letters 19(3), 299-307. https://doi.org/10.1111/ele.12568
Zimova, M., Hackländer, K., Good, J.M., Melo‐Ferreira, J.,
Alves, P.C., Mills, L.S. 2018. Function and underlying mechanisms of seasonal
colour moulting in mammals and birds: what keeps them changing in a warming
world?. Biological Reviews 93(3), 1478-1498. https://doi.org/10.1111/brv.12405
Zimova, M., Giery, S.T., Newey, S., Nowak, J.J., Spencer, M., Mills, L.S.
2020a. Lack of phenological shift leads to increased camouflage mismatch in
mountain hares. Proceedings of the Royal Society B 287(1941),
20201786. https://doi.org/10.1098/rspb.2020.1786
Zimova, M., Barnard, L.S., Davis, B.M., Kumar, A.V., Lafferty, D.J.R.,
Mills, L.S. 2020b. Using remote cameras to measure seasonal molts. Ecosphere
11, e03084. https://doi.org/10.1002/ecs2.3084
Zuur, A., Ieno, E.N., Smith, G.M. 2007. Analyzing
ecological data. Springer. https://doi.org/10.1007/978-0-387-45972-1
Zuur, A., Ieno, E.N., Walker, N., Saveliev, A.A., Smith, G.M. 2009. Mixed
effects models and extensions in ecology with R. Springer Science &
Business Media. https://doi.org/10.1007/978-0-387-87458-6
Annex / Anexo
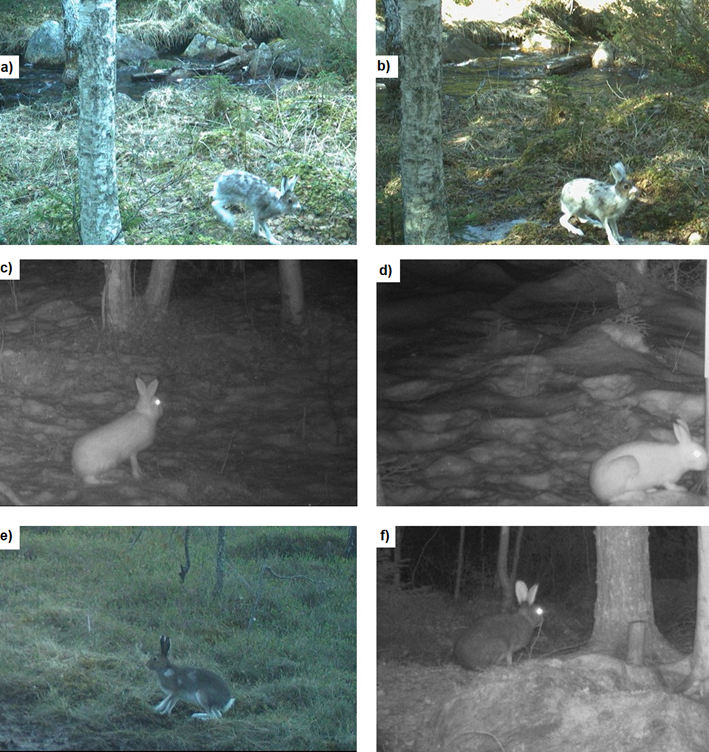
Figure A1. Images of the hares with the 3 fur colours: a) and b) moulting, c)
and d) white and e) and f) brown.
Figura A1. Imágenes de las liebres con los 3 colores
de pelaje. a) y b) mudando, c) y d) blanco y e) y f) marrón.
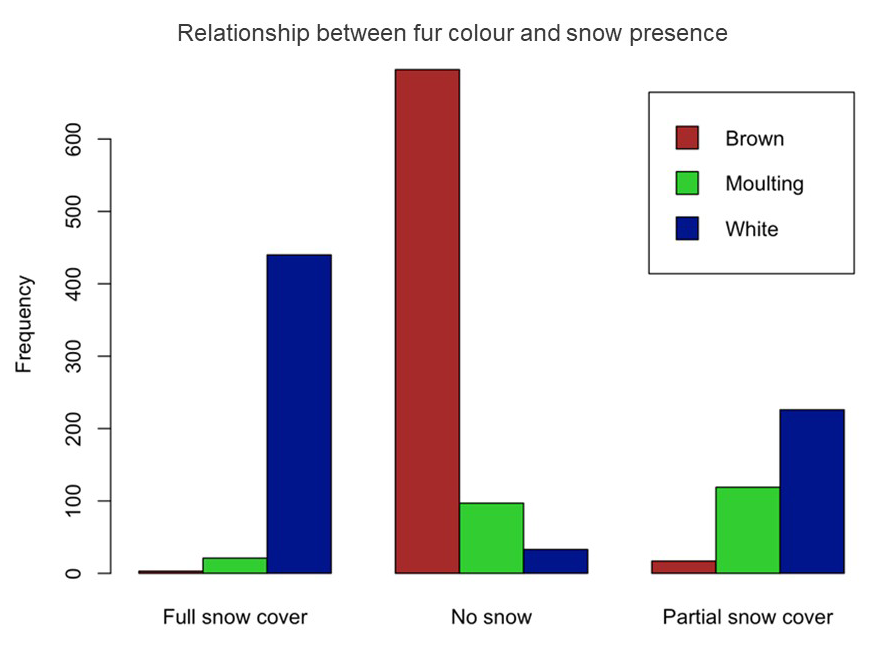
Figure A2. Relationship between fur color and snow presence.
Figura A2. Relaciòn entre color del pelaje y
presencia de nieve.
![]() , Davide Carniato2,*
, Davide Carniato2,* ![]() , Lars Hillstrom1
, Lars Hillstrom1 ![]() , Petter Hillborg1, Marcus Larsson3,
, Petter Hillborg1, Marcus Larsson3,![]() , Antonio J.
Carpio4
, Antonio J.
Carpio4 ![]()







