Introduction
Soil microorganisms are essential
components of ecosystem function and resilience (Graham et al. 2014). These
microorganisms play fundamental roles in key ecological processes such as
biogeochemical cycling, organic matter (OM) decomposition, carbon (C)
sequestration, net primary productivity, and natural regeneration (Zheng et al. 2019; Creamer et al. 2022). Among the great diversity of soil microorganisms, arbuscular
mycorrhizal fungi (AMF) play a crucial ecological role as widespread symbionts,
forming mutualistic associations with approximately 75% of plant species (Brundrett and Tedersoo 2018).
Traditionally, the assumed primary function
of AMF has been to enhance the uptake of water and macronutrients from the
soil, primarily phosphorus (P) and nitrogen (N), thereby benefiting the
survival and growth of their host plants (Smith and Read 2008; Smith and Smith 2011). This has led to extensive studies on the beneficial effects of
native AMF inoculum on growth indicators such as seedling height and biomass
production of agriculturally important crops such as Triticum spp. Zea
mays, Solanum lycopersicum, Solanum tuberosum and Coffea
arabica (Vosátka and
Gryndler 2000; Zhu et al. 2001; Al-Karaki 2006;
Begum et al. 2019; Perea-Rojas et
al. 2019; Abrar et al. 2024). Likewise, this
paradigm has overlooked the deeper exploration of other functions of this
fungal group in various ecological processes of ecosystems, such as OM
decomposition, soil structure, reproductive phenology of wild plants, and seed
germination. These processes become particularly relevant in the current
scenario of global changes such as climate change and soil desertification.
Through nutrient transport, AMF influences
the nutritional status of the host plant and its physiological ability to
respond to adverse environmental conditions. Therefore, these fungi are
recognized as buffers against environmental stress and protectors against
pathogen attack (Pozo et al. 2008). A meta-analysis by Veresoglou and Rillig (2012), which
analyzed 106 previous studies, found that AMF can reduce the severity of
disease, and that the efficacy of this suppression is highly dependent on the
specific identity of the AMF involved. For example, Funneliformis mosseae
is particularly effective in protecting against fungal pathogens, whereas Rhizoglomus
intraradices is particularly effective in protecting against
plant-parasitic nematodes. This perspective has promoted the study of AMF in
systems where environmental conditions are challenging, such as agricultural
areas, arid and semi-arid zones (Varela-Fregoso et al. 2017). In
contrast, AMF have been poorly studied in temperate forests, for example,
because it has long been assumed that the characteristic tree species of these
forests are exclusively associated with ectomycorrhizal fungi. This claim has
been challenged in recent studies, for example, Bueno et al. (2017) showed that arbuscular
mycorrhizal associations vary according to environmental gradients, such as
latitude and elevation, suggesting that our understanding of these associations
is still incomplete. Likewise, the involvement of AMF in the early stages of
the life cycle of tree species (Olivera-Morales et al. 2011) and in
ecological succession (Zobel
and Öpik 2014; Krüger et al. 2017) is now recognized.
The study of the multifunctionality of AMF
should be considered in future research, not only considering their role in
soil nutrient uptake and transport, but also their influence on other
ecological processes. This review aims to: i) deepen and highlight the
functions of AMF in key processes of terrestrial ecosystems, such as OM
decomposition, soil structuring and plant ecology (reproductive phenology and
seed germination), and ii) propose some ecological filters that influence the
composition of AMF. The study of the multifunctionality of these soil
microorganisms is urgent given the current conditions of deforestation and soil
desertification and the unprecedented alteration of terrestrial ecosystems.
Information search
For the review on the multifunctionality of
AMF, a literature search was conducted in interdisciplinary databases such as
Web of Science, Scopus and EBSCO. Specific keywords combined with Boolean
operators (AND, OR) were used to optimize the retrieval of relevant studies in
five main axes: (1) organic matter decomposition; (2) soil structure,
highlighting their influence on aggregate formation; (3) reproductive
phenology, evaluating their influence on flowering and fruiting of host plants;
(4) seed germination, considering their contribution to early root colonization
and mycorrhizal resistance; and (5) ecological filters regulating AMF activity,
such as elevation gradients, nutrient availability, host identity, and
anthropogenic disturbance. This strategy provides a comprehensive approach to
understanding the contribution of AMF to soil dynamics.
The search was conducted between March and
June 2024 and included publications from 1990 to 2024. A total of 208 articles
were initially retrieved. After applying inclusion and exclusion criteria, 117
articles were selected for detailed analysis. Inclusion criteria included
peer-reviewed articles in English or Spanish that presented empirical data or
comprehensive reviews that directly addressed at least one of the five axes of
AMF functionality. Exclusion criteria included duplicate entries,
non-peer-reviewed sources, publications with insufficient methodological
information, or studies focused exclusively on ectomycorrhizal fungi. This
process ensured a rigorous selection of literature
Arbuscular mycorrhizal fungi in OM decomposition
Early research on AMF suggested that they
had saprophytic activity, as they were found to colonize litter and proliferate
in various types of OM (Nicolson
1959; Gerdemann and Trappe 1974). This led to
the formulation of the 'direct mineral cycling' hypothesis proposed by Went and Stark (1968), which postulated the direct involvement of AMF in OM decomposition
and subsequent transport of nutrients to their host. However, this hypothesis
was rejected for lack of convincing evidence.
AMF lack the enzymatic machinery necessary
to degrade organic molecules (Tisserant
et al. 2013), so they only transport inorganic
forms of nutrients from soil to plants. This has been the paradigm for nutrient
flow from fungi to plants in the arbuscular mycorrhizal association. However,
this model is currently being challenged by studies using in vitro systems
showing that species of the genus Glomus assimilate organic forms of N (Hodge et al. 2001; Hodge and Storer
2015) and P (Koide and Kabir 2000). The role of AMF
extracellular enzymes in OM degradation is unclear, and recent findings on
access to organic forms of nutrients have been seriously questioned due to lack
of reproducibility and heterogeneity in the results obtained (Koide and Kabir 2000; Jansa et al. 2013). Furthermore, most experiments have focused on two specific
species, Rhizoglomus irregulare and Simiglomus hoi (Leigh et al. 2011), which limits inferences to higher taxonomic categories, as it is
unknown whether other species or genera exhibit similar behavior.
The colonization of AMF in litter (Bunn et al. 2019;
Kemmelmeier et al. 2022) and the proliferation of these fungi in some forms of OM (Gavito and Olsson 2003; Hodge and Fitter
2010; Barrett et al. 2011; Paterson et al. 2016) clearly indicate that AMF somehow intervene in decomposition and
even transfer some of the released nutrients to their host (Fig. 1) (Bunn et al. 2019).
However, the exact mechanisms remain unknown, as it is complex to establish the
direct involvement of these microorganisms, considering that they are
completely dependent on their host for C acquisition (Smith and Read 2008).
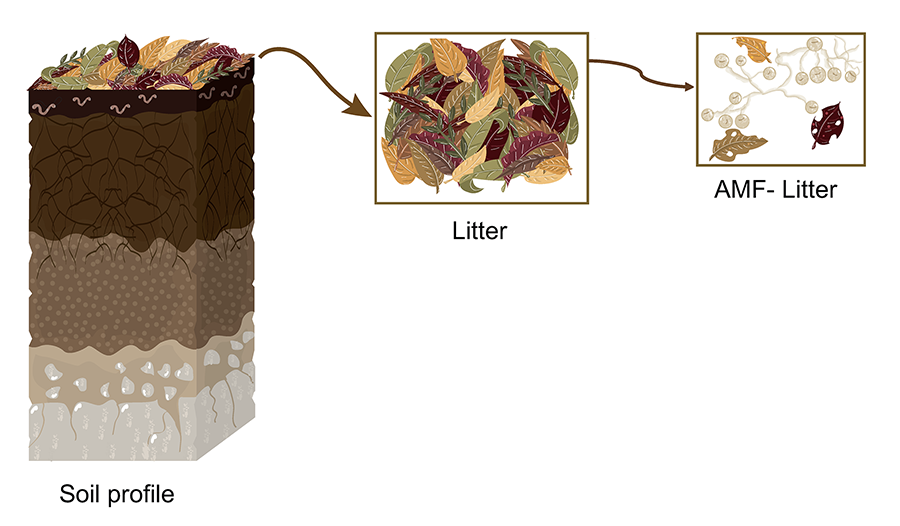
Figure 1. Schematic representation of a hypothetical soil profile
highlighting the litter layer and its progressive transformation, including
colonization by AMF (Arbuscular mycorrhizal fungi). The figure shows the
decomposition of plant litter and its interaction with AMF structures. Elaborated
by Vázquez-Santos LM (2024).
Figura 1. Representación de un perfil de
suelo hipotético en el que se resalta el mantillo y su transformación
progresiva, incluida la colonización por HMA (hongos micorrízicos arbusculares).
Se muestra la descomposición del tejido vegetal y su interacción con las
estructuras de HMA. Elaborado por Vázquez-Santos LM (2024).
The rapid growth of AMF extraradical
mycelium in OM patches can be interpreted as 'direct foraging' of these fungi
towards nutrient-rich sites (Welc
et al. 2010; Hodge 2014). It has been suggested that
AMF biochemically recognize nutrient patches and grow towards them through
chemical signaling mechanisms, similar to the pre-symbiotic recognition signals
between plant and fungus (Barrett
et al. 2011). However, it is also likely that the
hyphae randomly locate the patches and, once there, branch out into the
nutrient-rich zone (Barrett et
al. 2011).
While the direct involvement of these
microorganisms in OM decomposition is inconclusive, indirect mechanisms of
their involvement have been proposed. AMF hyphae can penetrate sites of
mineralization activity within the decomposing substrate (Jansa et al. 2013), facilitating the intervention of other decomposer microorganisms
such as bacteria. It has been suggested that 'hyper-symbionts' are transported
at the hyphal tips, physically decomposing the organic substrate and forming a
'secondary symbiosis' with the AMF (Jansa et al. 2013). At the same time,
fungi release C through hyphal exudates, stimulating microbial activity
associated with the hyphosphere, the zone of interaction between the hyphae and
the soil (Toljander et al.
2007).
The colonization of different soil habitats
may be an important ecological strategy of these fungi to increase their
exploration area, especially in topographically heterogeneous sites with low P
availability. Indeed, a recent study suggests that there is habitat selection
by certain AMF families. It was observed that Archeosporaceae were found
exclusively in litter, Paraglomeraceae in plant roots and Ambisporaceae in
mineral soil (Kemmelmeier et
al. 2022). The authors do not provide a conclusive
explanation for this pattern, but speculate that it is related to the
morphological (e.g., spore size, hyphal architecture, and root colonization
strategies) and functional traits (e.g., nutrient exchange efficiency, response
to environmental stress, and host specificity) of AMF that are conserved at the
family level (Hart and Reader
2002; Powell et al. 2009).
Arbuscular mycorrhizal fungi in soil
structure
OM improves the stability, porosity, and
water-holding capacity of soils because its presence promotes the formation and
stability of aggregates by acting as a cementing agent (Six et al. 2000).
Likewise, the mycelium of AMF acts as a cementing agent, binding soil particles
and promoting the formation of aggregates (macro [>250 µm] and micro [<
250 µm]) that are more resistant to erosion and increase porosity, which means
an increase in soil solution infiltration and gas exchange, and a decrease in
soil bulk density, which is crucial for the maintenance of agroecosystems
because it contributes to increasing the physical fertility of the soil (Fig. 2) (Lehmann et al. 2017; Fall et al. 2022; Wang et al. 2022).
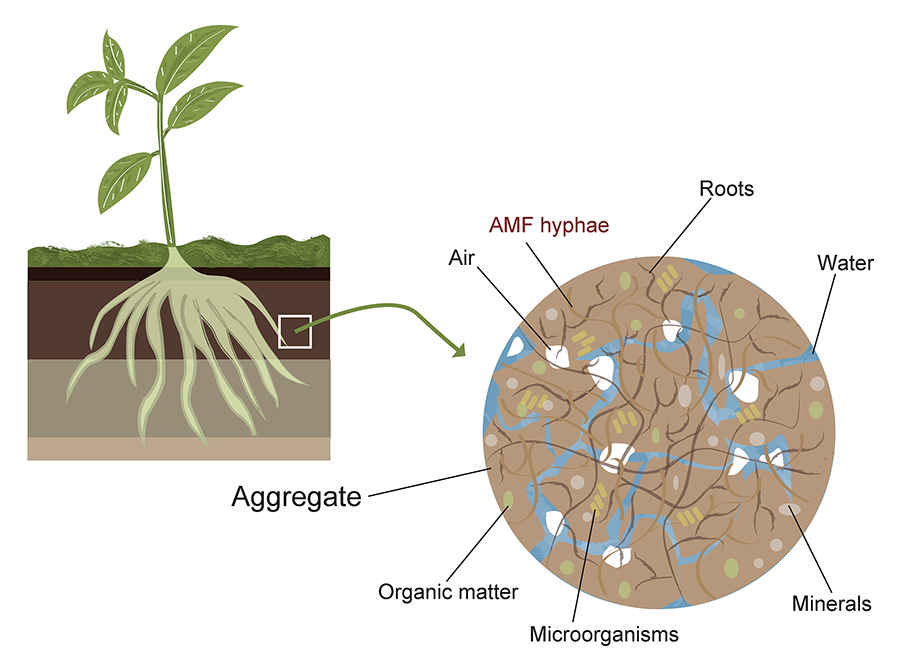
Figure 2. Example
of AMF (arbuscular mycorrhizal fungi) distribution in soil aggregates. The
figure illustrates the spatial organization of AMF within soil micro- and
macroaggregates, highlighting their hyphal networks, spore localization, and
potential interactions with soil organic matter and mineral particles. Elaborated
by Vázquez-Santos LM (2024).
Figura 2.
Ejemplo de distribución de HMA (hongos micorrícicos arbusculares) en los
agregados del suelo. Se ilustra la organización espacial de los HMA dentro de
los microagregados y macroagregados, destacando sus redes hifales, localización
de esporas, y potenciales interacciones con la materia orgánica del suelo y
partículas minerales. Elaborado por Vázquez-Santos LM (2024).
AMF have different strategies to colonize
the soil, which is reflected in the production of extraradical mycelium as a
result of the search for nutrients (Hart and Reader 2002, 2005). As well as they have different extraradical mycelial architecture
and thickness (Dodd et al. 2000; Drew et al. 2003; Beck et al. 2007), which affects differently in the aggregates ‘construction. On the
other hand, it has been reported that the process of soil aggregate formation
depends on the mycorrhizal species as well as the interaction with its host
plant (Piotrowski et al. 2004).
Because studies of AMF and saprophytic
fungi and their interactions with soil particles and other components of the
biota have been conducted destructively or in isolated photographs showing soil
aggregates and only one AMF species, various models have been proposed for how
the process of soil aggregate formation occurs (Rillig and Mummey 2006; Lehmann and
Rillig 2015; Jeewani et al. 2021). However, as
mentioned above, many species of AMF have distinct traits that are not
represented in such schemes. On the other hand, the stability of aggregates in
water as a product of mycorrhizal interactions is also carried out in a
disruptive manner, thus altering the relationship between hyphae, macro- and
microaggregates, and porous space (Kemper and Rosenau 1986; Jiménez-Martínez et al. 2024).
Jiménez-Martínez
et al. (2024) showed through micromorphological
studies, image analysis (with thin sections) and destructive techniques how Funneliformis
mosseae, Rhizophagus intraradices and Gigaspora gigantea
contribute differently to the formation of stable aggregates in water (modified
system) and how through thematic maps with high resolution mosaics (unmodified
system) they showed for one year how these mycorrhizal fungi contribute
differently to the formation of aggregates.
Therefore, it has been proposed as a
complementary technique to study the interactions between mycorrhizal fungi and
other components of the biota, such as bacterial communities (biofilms), as
well as processes of OM decomposition and how it relates to the processes of
formation of soil aggregates (Gutiérrez-Castorena et al. 2018; González-Vargas
and Gutiérrez-Castorena 2022; Jiménez-Martínez et al. 2024).
Arbuscular mycorrhizal fungi in the
reproductive phenology of the host
Experimental studies have shown that AMF
significantly improve nutrient uptake efficiency, particularly for P and N,
both of which are essential for plant metabolism and reproductive success (Derelle et al. 2015; Vosnjak et al. 2021). Wang and Tang
(2022) highlight that AMF influence key
reproductive traits, including flowering time, seed production, and fruit set,
ultimately enhancing the functional fitness of host plants. Their findings
suggest that AMF-mediated nutrient acquisition directly contributes to higher
reproductive efficiency, especially under nutrient-limited conditions.
In addition to their role in nutrient
uptake, AMF actively modulate plant phenology by modulating flowering and fruit
production (Vázquez-Santos
2019; Bennett and Meek 2020). Field studies in
temperate forests indicate that AMF colonization varies with the nutrient
requirements of plants during their reproductive stages, with significant
correlations between AMF root colonization and fruiting in species such as Acaena
elongata, Ageratina glabrata, and Solanum pubigerum (Vázquez-Santos 2025). Similarly, Vega-Frutis and Guevara (2009) reported
that higher AMF colonization during the dry season was associated with increased
flower and fruit production in Carica papaya, suggesting that AMF play
an important role in maintaining reproductive success under water-limited
conditions.
AMF affect flower and fruit production by modifying
hormone levels, promoting the accumulation of gibberellins and cytokinins, and
increasing nutrient and water uptake by the plant (Roman-García et al. 2004; Ludwig-Müller
2010). The study of reproductive phenology in
relation to AMF has hardly been addressed in wild plant species. However,
increased intraradical colonization of these fungi during the reproductive
period of their host has been observed in agriculturally important plant
species and semi-arid ecosystems (Bennett and Meek 2020). This suggests
that these fungi indirectly contribute to the reproductive success of plants
and may be a key component in the reproductive plasticity of their hosts in
different environments.
Mountains are ideal systems for comparing
the responses of organisms to the environment because of the presence of an elevation
gradient that produces variation in abiotic factors. In plant populations
established at lower elevations, the onset of the reproductive period (flower
bud burst) occurs earlier, and reproductive maturity is reached more slowly,
whereas in populations growing at higher elevations, flower bud burst is
delayed and flowers and fruits develop rapidly (Laiolo et al. 2015). Early flowering
results in reduced reproductive output due to frost damage to developing floral
tissues, a decrease in pollinators, intense herbivory, and lack of sufficient
resources (Hegland et al. 2009). At the same time, late flowering individuals are unable to
complete their reproductive cycles before the onset of adverse conditions such
as drought or frost (Anderson
et al. 2012). In this context, by increasing
nutrient uptake and water transport, AMF would allow the plant to buffer
adverse environmental conditions during its reproductive period along the elevation
gradient.
Variation in
plant reproductive response along the elevation gradient triggers changes in C
metabolism and microenvironmental conditions that affect AMF activity in roots
and soil. Patterns indicate that intraradical colonization and AMF diversity
are negatively correlated with elevation (Lugo et al. 2008; Gai et al. 2012; Vázquez-Santos et al. 2019). Thus, we can conclude that at higher
elevations, the high allocation of plant resources to the production of
reproductive structures, together with low temperature, high humidity and
acidic pH, limit intraradical colonization and AMF spore production. At the
same time, the low availability of nutrients at higher elevations favors the
maintenance of the association. At lower elevations, the colonization and
sporulation of a greater number of AMF species is favored by high plant C
transfer, low water availability, and warmer temperatures, which favor soil
microbial activity and, consequently, OM decomposition. The relationship
between reproductive phenology, AMF and elevation has not been proven and
represents an excellent field of study that would allow predicting the
variability of the arbuscular mycorrhizal association in the face of future
severe environmental changes, especially under the scenario of climate change.
Arbuscular mycorrhizal fungi in seed
germination
The AMF can directly and indirectly
influence seed germination by modulating soil conditions and biochemical
signals that trigger the germination process. It has been observed that seed
germination is higher in substrates with the presence of AMF propagules (Fig. 3) (spores,
previously infected roots). Their role goes beyond creating 'optimal' microenvironmental
conditions, as they also release signaling molecules and secondary metabolites,
such as strigolactones, that can stimulate seed germination in certain plant
species (Akiyama et al. 2005; Besserer et al.
2006). Strigolactones act as chemical signals in
the rhizosphere that promote hyphal branching in AMF and influence plant root
architecture, thereby facilitating early root colonization and enhancing
seedling establishment (Akiyama
et al. 2005). This suggests that these fungi may
induce seed germination (Koide 2000).
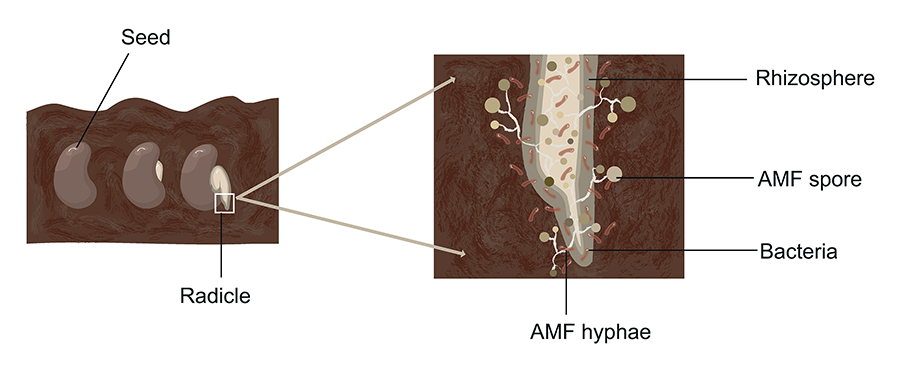
Figure 3. Illustration
of the early stages of root colonization by AMF (arbuscular mycorrhizal fungi) in
the rhizosphere. The image shows a germinating seed with its radicle surrounded
by AMF hyphae, spores, and associated rhizosphere bacteria. Elaborated
by Vázquez-Santos LM (2024).
Figura 3. Ilustración de las primeras etapas
de la colonización intra-radical por AMF (hongos micorrícicos arbusculares). La
imagen muestra una semilla en germinación con su radícula rodeada de hifas de
HMA, esporas y bacterias asociadas a la rizosfera. Elaborado
por Vázquez-Santos LM (2024).
The germination stage is crucial for the
establishment of an individual and is one of the most vulnerable in the plant
life cycle (Jurado and
Flores 2005). Therefore, the rapid establishment of
the arbuscular mycorrhizal association will allow the seedling to obtain the
necessary nutrients for its survival, growth and development. Experimental
studies have shown that AMF have a positive effect on seed germination. Vázquez-Santos et al. (2024) found that species such as Acaena elongata, Ageratina
glabrata, and Solanum pubigerum exhibited higher germination
percentages when inoculated with AMF. Similarly, Olivera-Morales et al. (2011) reported that AMF inoculation increased germination and seedling
establishment of Quercus rugosa in a temperate forest in Mexico. Research
by Ballina-Gómez et al.
(2017) demonstrated that the interaction between
AMF and light availability significantly increased germination rates in tree
species from tropical dry forests, such as Tecoma stans, Sapindus
saponaria, and Bauhinia forficata. Additionally. These findings
suggest that the presence of AMF not only facilitates germination but also
enhances seedling establishment and survival, making them a key biotic factor
in plant recruitment and restoration strategies.
The role of AMF in seed germination and
seedling establishment can also be analyzed from another perspective: their
contribution to plant defense through mycorrhizal-induced resistance (MIR), a
priming effect that enhances the ability of seedlings to respond more
efficiently to biotic and abiotic stresses (Pozo and Azcón-Aguilar 2007). This
priming effect may play an important role in seed germination by modulating
signaling pathways such as jasmonic acid and salicylic acid, which are crucial
for plant stress responses. Activation of these pathways could increase the
likelihood of successful germination and early seedling establishment by
enhancing pathogen resistance and optimizing resource allocation during this
critical developmental stage (Pozo
and Azcón-Aguilar 2007). These findings highlight
the potential dual role of AMF, not only as facilitators of optimal germination
conditions, but also as biological enhancers that prepare the seedling for
environmental challenges, ultimately improving plant recruitment and survival
under dynamic ecological conditions.
Bacteria and other organisms associated
with the AMF hyphosphere (Jansa et
al. 2013) could act as scarifying agents for seeds,
thus enhancing their germination in association with these fungi. Understanding
the role of AMF in seed germination is an important step that will elucidate
the function of these fungi in forest natural regeneration. In addition, it is
important to understand whether these microorganisms represent an adaptive
strategy of plants that favors their germination and establishment in
topographically heterogeneous sites.
Ecological filters that affect
arbuscular mycorrhizal fungi
AMF are subject to various ecological
filters that influence their distribution, diversity and functionality within
ecosystems. These filters can be natural, such as elevation gradients, the
availability of essential nutrients and the biological identity of the host
plant, or anthropogenic, resulting from human activities such as deforestation,
agricultural intensification, and land-use change that alter the composition
and structure of AMF communities (Chagnon et al. 2021). On a global scale,
climate change is altering temperature and precipitation patterns, affecting
the functionality of these fungi and their interactions with plants (Alguacil et al. 2021). Ecological filters act as selection mechanisms that determine
which species colonize a given habitat, a concept extensively explored by Zobel (2016), who
analyzes the interaction between species, environmental filters, and functional
traits in community structuring. Future research on ecological filters is
essential to predict how AMF will respond to environmental change, and to
develop conservation and management strategies that ensure their ecological
role in ecosystems.
Natural filters: elevation
gradient
Along the elevation gradient, there are
changes in temperature and precipitation that result in differences in soil
properties. As elevation increases, temperature and pH decrease and soil
moisture increases (Chen et al.
2017). Nutrient availability is deficient at higher
elevations due to low enzymatic and mineralization activity caused by low
temperatures, resulting in an apparent accumulation of litter (Fahey et al. 2015; Laiolo et al. 2015). An inverse pattern is found at lower elevations where temperature
is warmer, pH is less acidic, soil moisture decreases, and microbial activity
increases (Fahey et al. 2015; Laiolo et al. 2015).
AMF respond significantly to these
elevation changes. Studies conducted in different mountains have shown that AMF
diversity and colonization tend to decrease with increasing elevation due to
harsher conditions and lower nutrient availability (Guo et al. 2020). At lower elevations,
where conditions are more favorable for microbial activity, AMF colonization
and diversity tend to be higher. For example, on Mount Taibai in China, AMF
diversity peaked at intermediate elevations and declined at the highest and
lowest elevations, suggesting optimal adaptation to the edaphic conditions of
intermediate elevations (Zhang et
al. 2022). Another study on Mt. Helen in the UU EE
showed that the structure of the AMF community varied significantly with elevation
and was influenced by factors such as soil pH, organic matter content, and
nutrient availability (Guo et al.
2020). Therefore, the effect of elevation gradient
on AMF activity is governed by the microenvironmental changes resulting from
the interaction of climate, topography, relief, and elevation.
Nutrient availability
Soil nutrient availability has a
significant impact on AMF colonization and diversity (Johnson 2010). P
is a key nutrient that directly affects AMF colonization. In soils with low
phosphorus availability, plants tend to rely more on AMF for the uptake of this
nutrient. This is because AMF have the ability to explore larger volumes of
soil than roots to access P reserves. One study showed that under low P
conditions, mycorrhizal colonization and extraradical mycelium length increased
significantly, thereby improving plant nutrition (Akter et al. 2024; Xiao et al. 2023).
Nitrogen availability also plays a critical
role. However, the effect of N can be twofold. Studies have shown that high N
availability can reduce plant dependence on AMF, thereby reducing mycorrhizal
colonization. This occurs because plants can obtain sufficient N directly from
the soil without the need to establish energetically costly symbiotic
associations. However, in soils with limited N, plants increase their
dependence on AMF, which can increase the colonization and diversity of these
fungi in the soil (Fall et al. 2022).
It is important to clarify that this
interaction is not always beneficial for the plant. Johnson (2010) proposes the functional
equilibrium model, which suggests that a high concentration of N and P in the
soil can have an antagonistic effect on the plant-fungus relationship. In this
model, a high concentration of N and low P strengthens the mycorrhizal association
because plants are more dependent on fungi for P uptake. On the other hand, a low
N and high P can turn the interaction into a commensal one, where the benefits
to the plant are significantly reduced.
The consideration of other macronutrients
besides P and N, such as potassium (K), is essential in the study of the
arbuscular mycorrhizal association. The K is essential for osmotic regulation
and enzyme activation in plants, but the role of AMF in potassium transfer is
poorly understood. Plants also take up these essential nutrients for growth and
development. Similarly, micronutrients such as zinc (Zn) and iron (Fe) are
essential for many enzymatic and structural functions in plants (Xiao et al. 2023).
The transfer of these other nutrients by AMF remains largely unexplored and an
active area of research.
Plant biological identity
Plant biological traits, such as root
structure, leaf area, and life form, have a significant impact on AMF
colonization and function (Davison
et al. 2011). Plant biological identity acts as an
important ecological filter, determining the degree of "functional
compatibility" between fungi and plants through specific biochemical and
physiological interactions (Chagnon
et al. 2013). Recent studies suggest that these
associations are not random but rather regulated by factors that influence the
efficiency and specificity of the symbiosis.
Root architecture strongly influences AMF
colonization. Plants with fine, highly branched roots, such as grasses, tend to
support higher AMF diversity and colonization rates than species with thicker,
less branched roots (Morris et
al. 2013; Li et al. 2023). Studies in temperate
and subtropical grasslands have shown that species with fibrous root systems
have more colonization sites, allowing for greater diversity of AMF taxa (Garrido et al. 2023). In addition, root longevity and turnover rates influence the
persistence and structure of AMF networks, reinforcing the role of plant
functional traits in symbiotic efficiency (Sepp et al. 2009).
Leaf traits, particularly plant leaf area
and photosynthetic capacity, also influence the AMF interactions. Plants with
larger leaf areas and higher photosynthetic rates allocate more carbohydrates
to AMF, supporting higher colonization rates and improved nutrient uptake (Li et al. 2023). A
study conducted in a subtropical forest found that plant species with larger
leaf areas and higher photosynthetic rates had more intense AMF colonization in
their roots (Li et al. 2023).
Plant life form, whether trees, shrubs,
herbs, or epiphytes, also plays an important role in the diversity and
structure of AMF communities. Trees, for example, tend to host a more diverse
AMF assemblage than herbs and shrubs due to their longer root life, deeper
rooting systems, and more stable microenvironmental conditions (Chen et al. 2020).
Recent research using multilayer network analysis has shown that plant-AMF
interactions follow specific structural patterns, with plant species
differentially filtering AMF based on their functional traits (Garrido et al. 2023). These findings highlight the importance of understanding plant
functional diversity is crucial for predicting AMF community structure and
ecosystem functioning.
Anthropogenic filters
Nutrient availability, plant community
structure, and AMF activity in soils are affected by anthropogenic activities (Dickie et al. 2013). The magnitude of these changes depends on the intensity,
frequency, and type of disturbance (Teste and Dickie 2017; van der Heyde et al. 2017). Low-intensity disturbances, such as selective weeding, minimally
alter the physical properties (e.g., structure and bulk density) and chemical
properties (e.g., nutrient availability and pH), while promoting gradual
changes in plant community composition that largely preserve native plant
species (Sánchez-Fuente 2019). However, even mild disturbance can lead to shifts in AMF community
structure, decreasing species richness and favoring the proliferation of AMF
taxa that are less sensitive to environmental changes in soil properties (Schnoor et al. 2011; Brundrett
and Ashwath 2013). Changes in richness may not be
observed, but there may be changes in abundance of some AMF species (Fig. 4) (Violi et al. 2008).
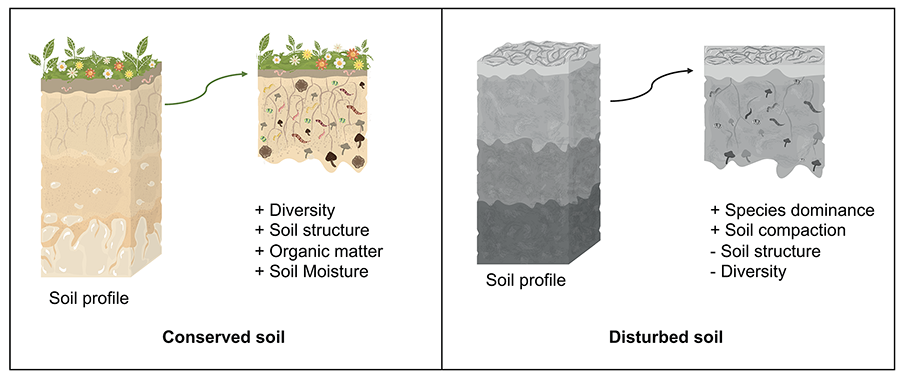
Figure 4. Arbuscular
mycorrhizal fungi in a preserved soil vs. a disturbed soil. Prepared
by Vázque-Santos LM (2024).
Figura 4. Hongos
micorrícicos arbusculares en un suelo conservado frente a un suelo alterado. Elaborado por Vázque-Santos LM (2024).
High-intensity disturbances, such as
intensive agriculture and deforestation, exert strong selective pressures on
AMF communities, leading to phylogenetic and trait-based filtering (Chagnon et al. 2022). Long-term agricultural practices tend to favor AMF species with
specific functional traits, particularly those adapted to disturbed
environments, while reducing overall community diversity. These findings are
consistent with the evolutionary framework proposed by Verbruggen and Kiers (2010), which suggests that anthropogenic management practices drive
selection for AMF species with traits that enhance their persistence in
intensively managed soils.
Chronic disturbance also shapes plant
community dynamics, favoring species with disturbance-tolerant traits that
dominate ecosystems (Mayfield
et al. 2010). As a result, physical, chemical, and
biological properties of soil are negatively altered, leading to functional
degradation (Mayfield et al.
2010). Plants that successfully colonize degraded
soils tend to be strong competitors for resources (Ramos-Zapata et al. 2013), and their mycorrhizal associations can significantly influence
local AMF composition. Frequently, more degraded sites have lower alpha and
beta diversity compared to less affected areas, highlighting the cascading
effects of ecosystem degradation on AMF networks (García et al. 2018). Furthermore,
prolonged disturbance may weaken the mutualistic balance of the symbiosis,
reducing the benefits provide by AMF to plant hosts and shifting interactions
towards more neutral or even parasitic associations (Verbruggen and Kiers 2010).
The life history strategies of both AMF
species and their plant hosts determine the colonization success and
persistence of species in degraded sites. Chagnon et al. (2013) propose that
severe anthropogenic disturbance favors the presence of generalist AMF species,
which tolerate high levels of disturbance and have rapid growth and early
investment in the formation of large numbers of small spores. The family
Glomeraceae has been classified as predominantly generalist (Klironomos and Hart 2002; Hart and Reader
2005). However, recent studies indicate a
significant diversity of life history traits within Glomeraceae, comparable to
the variation observed in other AMF families (Horsch et al. 2023). In this context, Davison et al. (2011) suggest that plant species with similar functional traits associate
with functionally analogous AMF taxa, i.e., generalist plants tend to form
symbioses with generalist AMF species. However, more evidence is needed to
support this hypothesis.
Arbuscular mycorrhizal fungi in
climate change
Rising temperatures, shifts in
precipitation regimes, and elevated carbon dioxide (CO₂)
concentrations are well recognized abiotic factors that influence plant
reproductive phenology (Beaubien
and Hamann 2011; Zhang et al. 2021). These extreme
weather events characteristics of global climate change are expected to drive
local changes in plant phenology, species distributions, extinctions, and
disruptions in above- and belowground biotic interactions (Alberton et al. 2005). For example, earlier flowering and fruiting may negatively affect
plant-pollinator and plant-disperser interactions, leading to cascading effects
on ecosystem functionality (Haggerty and Galloway 2011).
Plant species will need to adapt in situ to
climate change, and their association with AMF may provide a key advantage due
to their roles in nutrient and water transfer, organic matter decomposition,
pathogen protection, and temperature tolerance. AMF can also adapt rapidly to
environmental changes, helping both fungi and their host plants to mitigate the
effects of climate change. Experimental studies indicate that elevated CO₂
levels increase intraradical colonization and AMF biomass in soils, as plants allocate
more photosynthates to fungal symbionts (Alberton et al. 2005). This suggests
that AMF symbiosis may increase soil carbon sequestration because these fungi
contribute to carbon storage through the production of glomalin, a highly
recalcitrant, carbon-rich protein (Drigo et al. 2010).
However, climate change is not uniformly
beneficial to AMF communities. Increased temperatures and altered precipitation
patterns can reduce soil phosphorus (P) availability (Hou et al. 2018), limiting nutrient
exchange in AMF-host interactions. In this scenario, AMF may enhance plant
tolerance to heat stress and improve phosphorus uptake (Zhu et al. 2017).
However, when climatic conditions exceed AMF tolerance thresholds, fungal
biomass, intraradical colonization, and spore production decrease, negatively
affecting plant-mycorrhizal mutualisms (Zhu et al. 2017).
Further evidence suggests that the effects
of climate change on AMF communities are highly context dependent. Weber et al. (2019) found that AMF responses to simultaneous drivers of global
change-including increases in temperature, CO₂ levels, and
nitrogen deposition-were highly variable. Their study highlighted
species-specific and ecosystem-dependent differences in AMF functional traits,
suggesting that some AMF taxa may be more resilient to climate change than
others. Similarly, Alguacil et
al. (2021) examined AMF responses to simulated
climate warming and drying and demonstrated contrasting effects among AMF
families, with some taxa benefiting from warmer conditions while others
declined. In addition, Solaiman
(2024) emphasized that AMF plays a critical role in
organic matter decomposition and soil carbon sequestration, reinforcing their
potential role in climate change mitigation.
These findings highlight the need for a
deeper understanding of how AMF diversity, functional traits, and ecological
roles shift under climate stressors. Given the complex responses of AMF to
multiple drivers of global change, future research should focus on identifying
resilient AMF species, exploring their functional plasticity, and assessing
their potential for ecosystem restoration in climate-altered landscapes.
Main challenges in the study of
arbuscular mycorrhizal fungi
The SARS-CoV-2 pandemic in 2019 has
affected the production of scientific research, including studies on AMF. As result,
there is urgent need to integrate and synthesize existing data to compensate
for research gaps and provide a clear understanding of AMF ecology. However,
beyond pandemic-related disruptions, several key challenges persist in AMF
research.
One major challenge is understanding how global
climate change affects AMF functionality. These fungi play an important role in
plant adaptation to stress conditions such as prolonged drought, high
temperatures, and soil degradation. Additionally, AMF enhance carbon capture
and sequestration, contributing to climate change mitigation. However, it is
unknown how increased temperatures, changes in precipitation regimes, and
changes in soil factors will affect the functionality of AMF. Moreover, the
functional diversity of AMF across ecosystems is still poorly understood,
making it difficult to predict how these fungi will respond to environmental
stressors and ecosystem disruptions.
To address these gaps, meta-analysis serves
as a powerful tool to synthesize results from multiple studies, increase
statistical power and sample size, and identify consistent ecological patterns
across diverse environments. By integrating data from independent studies,
meta-analyses can provide stronger evidence of causal relationships and offer
deeper insights into how AMF respond to climate change and anthropogenic
pressures. In the context of AMF research, meta-analyses can clarify the
effects of environmental variables on colonization, diversity, and
functionality, helping to provide a stronger foundation for future studies and
practical applications in ecosystem management.
Conclusions
Arbuscular mycorrhizal fungi are
multifunctional soil microorganisms that play a crucial role in ecological
processes. They facilitate the OM decomposition, mediate vegetation structure,
enhance nutrient transport, and mitigate environmental stress during the
reproductive period of their host plants. They also promote seed germination
and contribute to soil formation and stability by forming and strengthening
aggregates.
Arbuscular mycorrhizal symbiosis is a key
ecological strategy that enables plants to adapt to environmental change, from
localized disturbances such as soil degradation to broader challenges such as
global climate change.
Despite significant advances in the
understanding of AMF ecology and function, several knowledge gaps remain.
Future research should focus on quantifying AMF contributions to carbon
sequestration, elucidating their interactions with other soil microbiota, and
exploring their potential in large-scale ecological restoration projects. In
addition, investigating the functional diversity of AMF under different
environmental conditions will provide valuable insights into their adaptability
and effectiveness in addressing emerging ecological challenges.
Author contributions
Yasmin Vázquez: Conceptualization,
Investigation; Yasmin Vázquez, Silvia Castillo and Arturo Martínez:
writing-original draft, writing – review and editing
Data accesibility
Requests for data can be made directly to
the author for correspondence by mail.
Financing, required permits, potential
conflicts of interest and acknowledgments
This research did not receive any specific
grants from funding agencies in the public, commercial or non-profit sectors. We declare that we have no conflicts of interest.
This research is part of the doctoral
studies of Y. Vázquez-Santos in the Posgrado en Ciencias Biológicas of the
Universidad Nacional Autónoma de México. Y. Vázquez-Santos acknowledges
the Secretaría de Ciencia, Humanidades, Tecnología e Innovación
(SECIHTI)-Mexico (No. 818569) for scholarships to pursue her doctoral studies. Vázquez-Santos LM for the preparation of the illustrative material.
Martínez-Orea Y for revision and translation.
References
Abrar, M., Zhu, Y., Rehman, M.M.U., Batool, A., Duan, H.X., Ashraf, U.,
Xiong, Y.C. 2024. Functionality of arbuscular
mycorrhizal fungi varies across different growth stages of maize under drought
conditions. Plant Physiology and Biochemistry 213, 108839. https://doi.org/10.1016/j.plaphy.2024.108839
Akiyama, K, Matsuzaki, K.I., Hayashi, H. 2005. Plant
sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi.
Nature 435(7043), 824-827. http://doi.org/10.1038/nature03608
Akter, S., Kamruzzaman, M., Sarder, M.P., Amin,
M.S., Joardar, J.C., Islam, M.S, Halder, M. 2024. Mycorrhizal fungi increase
plant nutrient uptake, aggregate stability and microbial biomass in the clay
soil. Symbiosis 1–14.
https://doi.org/10.1007/s13199-024-00994-4
Al-Karaki, G.N. 2006. Nursery inoculation of tomato with arbuscular
mycorrhizal fungi and subsequent performance under irrigation with saline
water. Scientia Horticulturae 109(1):1–7. https://doi.org/10.1016/j.scienta.2006.02.019
Alberton, O., Kuyper, T.W., Gorissen, A. 2005. Taking mycocentrism
seriously: mycorrhizal fungal and plant responses to elevated CO2. New
Phytologist 167(3):859–868. https://doi.org/10.1111/j.1469-8137.2005.01458.x
Alguacil, M.M., Schlaeppi, K., López-García, Á., van der Heijden, M.G.A.,
Querejeta, J.I. 2021. Contrasting Responses of Arbuscular Mycorrhizal Fungal
Families to Simulated Climate Warming and Drying in A Semiarid Shrubland. Microbial
Ecology 0123456789: 1–4. https://doi.org/10.1007/s00248-021-01886-6
Anderson, J.T., Inouye, D.W., McKinney, A.M., Colautti, R., Mitchell-Olds,
T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing
flowering phenology in response to climate change. Proceedings of the Royal
Society 282:3843–3852. https://doi.org/10.1098/rspb.2012.1051
Ballina-Gómez, H.S., Ruiz-Sánchez, E., Ambriz-Parra, E., Alvarado-López, C.J.
2017. Efecto de la luz y micorrizas en la germinación de semillas de
árboles de selvas secas. Madera y bosques 23(3):29-37. https://doi.org/10.21829/myb.2017.2331531
Barrett, G., Campbell, C.D., Fitter, A.H., Hodge, A. 2011. The arbuscular
mycorrhizal fungus Glomus hoi can capture and transfer nitrogen from
organic patches to its associated host plant at low temperature. Applied
Soil Ecology 48:102–105. https://doi.org/10.1016/j.apsoil.2011.02.002
Beck, A., Haug, I., Oberwinkler, F., Kottke, I. 2007. Structural
characterization and molecular identification of arbuscular mycorrhiza
morphotypes of Alzatea verticillata (Alzateaceae), a prominent tree in the
tropical mountain rain forest of South Ecuador. Mycorrhiza 17:607-625. https:/doi.org/10.1007/s00572-007-0139-0
Beaubien, E.,
Hamann, A. 2011. Spring flowering response to
climate change between 1936 and 2006 in Alberta, Canada. Bioscience
61:514–524. https://doi.org/10.1525/bio.2011.61.7.6
Begum, N., Ahanger, M.A., Su, Y., Lei, Y.,
Mustafa, N.S.A., Ahmad, P., Zhang, L. 2019. Improved drought tolerance by AMF
inoculation in maize (Zea mays) involves physiological and biochemical
implications. Plants 8(12):579. https://doi.org/10.3390/plants8120579
Bennett, A.E.,
Meek, H.C. 2020. The influence of arbuscular
mycorrhizal fungi on plant reproduction. Journal of chemical ecology
46(8):707–721. https://doi.org/10.1007/s10886-020-01192-4
Besserer, A., Puech-Pagès, V., Kiefer, P., Gomez-Roldan, V., Jauneau, A.,
Roy, S., Séjalon-Delmas, N. 2006. Strigolactones stimulate arbuscular
mycorrhizal fungi by activating mitochondria. PLoS biology 4(7):e226.
https://doi.org/10.1371/journal.pbio.0040226
Brundrett,
M.C., Ashwath, N. 2013. Glomeromycotan mycorrhizal
fungi from tropical Australia III. Measuring diversity in natural and disturbed
habitats. Plant Soil 370:419–433. https://doi.org/10.1007/s11104-013-1613-4
Brundrett, C.M., Tedersoo, L. 2018. Evolutionary history of mycorrhizal
symbioses and global host plant diversity. New Phytologist
220:1108–1115. https://doi.org/10.1111/nph.14976
Bueno, C.G., Moora, M., Gerz, M., Davison, J., Öpik, M., Pärtel, M.,
Zobel, M. 2017. Plant mycorrhizal status, but not type, shifts with latitude
and elevation in Europe. Global Ecology and Biogeography 26(6):690-699. https://doi.org/10.1111/geb.12582
Bunn, R. A., Simpson, D.T., Bullington, L.S., Lekberg, Y., Janos, D.P.
2019. Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular
mycorrhizal fungi colonize leaf litter, but why? ISME Journal
13:1891–1898. https://doi.org/10.1038/s41396-019-0403-2
Chagnon, P.L., Bradley, R.L., Maherali, H., Klironomos, J.N. 2013. A
trait-based framework to understand life history of mycorrhizal fungi. Trends
in plant science 18(9):484–491. https://doi.org/10.1016/j.tplants.2013.05.001
Chagnon, P.-L., Bradley, R.L., Lafond, J., Maxime, Paré, C., Penaud, V.,
Bradley, R.L., Penaud, V., Lafond, J., Paré, M.C. 2021. Trait-based and
phylogenetic filtering of arbuscular mycorrhizal fungal communities under
long-term agricultural practices. Plant and Soil. https://doi.org/10.1007/s11104-021-05155-w
Chagnon, P.L., Bradley, R.L., Lafond, J., Paré, M.C., Penaud, V. 2022.
Trait-based and phylogenetic filtering of arbuscular mycorrhizal fungal
communities under long-term agricultural practices. Plant and Soil 1-15.
https://doi.org/10.1007/s11104-021-05155-w
Chen, D., Yu, M., González, G., Zou, X., Gao, Q. 2017. Climate impacts
on soil carbon processes along an elevation gradient in the tropical Luquillo
experimental forest. Forests 8:90. https://doi.org/10.3390/f8030090
Chen, W., Koide, R.T., Eissenstat, D.M. 2020.
Topographic and Host Effects on Arbuscular Mycorrhizal and Ectomycorrhizal
Fungal Communities in a Forested Watershed. Ecosystems, 23:1537–1546. https://doi.org/10.1007/s10021-020-00486-8
Creamer, R.E., Barel, J.M., Bongiorno, G., Zwetsloot, M.J. 2022. The life of
soils: Integrating the who and how of multifunctionality. Soil Biology and
Biochemistry 166:108561. https://doi.org/10.1016/j.soilbio.2022.108561
Davison, J., Öpik, M., Daniell, T.J., Moora, M., Zobel, M. 2011. Arbuscular
mycorrhizal fungal communities in plant roots are not random assemblages. FEMS
Microbiology Ecology 78:103-115. https://doi.org/10.1111/j.1574-6941.2011.01103.x
Derelle, D., Courty, P.E., Dajoz, I., Declerck, S., van Aarle, I.M.,
Carmignac, D., et al. 2015. Plant identity and density can influence arbuscular
mycorrhizal fungi colonization, plant growth, and reproduction investment in
coculture. Botany 93:405–412. https://doi.org/10.1139/cjb-2014-018
Dickie, I.A., Martínez-García, L.B., Koele, N., Grelet, G.A., Tylianakis,
J.M., Peltzer, D.A., Richardson, S.J. 2013. Mycorrhizas and mycorrhizal fungal
communities throughout ecosystem development. Plant and Soil
367(1):11–39. https://doi.org/10.1007/s11104-013-1609-0
Dodd, J.C., Boddington, C.L.,
Rodriguez, A., Gonzalez-Chavez, C., Mansur, I. 2000. Mycelium
of arbuscular mycorrhizal fungi (AMF) from different genera: form, function and
detection. Plant and soil 226:131-151.
Drew, E.A., Murray, R.S., Smith, S.E., Jakobsen, I. 2003. Beyond the
rhizosphere: growth and function of arbuscular mycorrhizal external hyphae in
sands of varying pore sizes. Plant and Soil 251:105-114.
Drigo, B., Pijl, A.S., Duyts, H., Kielak, A.M., Gamper, H.A., Houtekamer,
M.J., Kowalchuk, G.A. 2010. Shifting carbon flow from roots into
associated microbial communities in response to elevated atmospheric CO2.
Proceedings of the National Academy of Sciences, 107:10938–10942. https://doi.org/10.1073/pnas.0912421107
Fahey, T.J., Sherman, R.E., Tanner, E.V. 2015. Tropical montane
cloud forest: environmental drivers of vegetation structure and ecosystem
function. Journal of Tropical Ecology 32:355–367. https://doi.org/10.1017/S0266467415000176
Fall, A.F., Nakabonge, G., Ssekandi, J.,
Founoune-Mboup, H., Apori, S.O., Ndiaye, A., Ngom, K. 2022. Roles of arbuscular
mycorrhizal fungi on soil fertility: Contribution in the improvement of
physical, chemical, and biological properties of the soil. Frontiers in
Fungal Biology 3. https://doi.org/10.3389/ffunb.2022.723892
Gai, J.P., Tian, H., Yang, F.Y., Christie, P., Li, X.L., Klironomos,
J.N. 2012. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation
gradient. Pedobiologia 55:145–151. https://doi.org/10.1016/j.pedobi.2011.12.004
García, D., Davison, J., Moora, M., Öpik, M., Feng, H., Hiiesalu, I.,
Zobel, M. 2018. Anthropogenic disturbance equalizes diversity levels in
arbuscular mycorrhizal fungal communities. Global Change Biology
24(6):2649–2659. https://doi.org/10.1111/gcb.14131
Garrido, J.L., Alcántara, J.M.,
López‐García, Á., Ozuna,
C.V., Perea, A.J., Prieto, J., Azcón‐Aguilar,
C. 2023. The structure and ecological function of the
interactions between plants and arbuscular mycorrhizal fungi through multilayer
networks. Functional Ecology 37(8):2217-2230. https://doi.org/10.1111/1365-2435.14378
Gavito, M.E.,
Olsson, P.A. 2003. Allocation of plant carbon to
foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiology
Ecology 45:181–187. https://doi.org/10.1016/S0168-6496(03)00150-8
Gerdemann, J.W.,
Trappe, J.M. 1974. The Endogonaceae in the Pacific
Northwest. Memoir No. 5. The New York Botanical Garden, New York.
González-Vargas, T., Gutiérrez-Castorena,
M.D.C. 2022. Brightness Values-Based Discriminant Functions for Classification
of Degrees of Organic Matter Decomposition in Soil Thin Sections. Spanish
Journal of Soil Science 12:10348. https://doi.org/10.3389/sjss.2022.10348
Graham, E.B., Wieder, W.R., Leff, J.W., Weintraub, S.R., Townsend, A.R.,
Cleveland, C.C., et al. 2014. Do we need to understand microbial communities to
predict ecosystem function? A comparison of statistical models of nitrogen
cycling processes. Soil Biology and Biochemistry 68:279–282. https://doi.org/10.1016/j.soilbio.2013.08.023
Guo, Y., Ren, C., Yi, J., Doughty, R., Zhao, F. 2020. Contrasting
responses of rhizosphere bacteria, fungi and arbuscular mycorrhizal fungi along
an elevational gradient in a temperate montane forest of China. Frontiers
in Microbiology 11:514865. https://doi.org/10.3389/fmicb.2020.02042
Gutiérrez‐Castorena, M.C., Gutiérrez‐Castorena, E.V., González‐Vargas, T., Ortiz‐Solorio, C.A., Suástegui‐Méndez, E., Cajuste‐Bontemps, L., Rodríguez‐Mendoza, M.N. 2018. Thematic micro‐maps of soil components using high‐resolution
spatially referenced mosaics from whole soil thin sections and image analysis. European
Journal of Soil Science 69: 217-231. https://doi.org/10.1111/ejss.12506
Haggerty,
B.P., Galloway, L.F. 2011. Response of individual
components of reproductive phenology to growing season length in a monocarpic
herb. Journal of Ecology 99:242–253. https://doi.org/10.1111/j.1365-2745.2010.01744.x
Hart, M.M., Reader, R.J. 2002. Does percent root length colonization and
soil hyphal length reflect the extent of colonization for all AMF? Mycorrhiza
12:297–301. https://doi.org/10.1007/s00572-002-0186-5
Hart, M.M.,
Reader, R.J. 2005. The role of the external
mycelium in early colonization for three arbuscular mycorrhizal fungal species
with different colonization strategies. Pedobiologia 49: 269–279. https://doi.org/10.1016/j.pedobi.2004.12.001
Hegland, S.J., Nielsen, A., Lázaro, A., Bjerknes, A.L., Totland, Ø. 2009.
How does climate warming affect plant-pollinator interactions? Ecology
Letters 12:184–195. https://doi.org/10.1111/j.1461-0248.2008.01269.x
Hodge, A. 2014. Interactions between arbuscular mycorrhizal fungi and
organic material substrates. Advances in applied microbiology 89:47–99.
https://doi.org/10.1016/B978-0-12-800259-9.00002-0
Hodge, A., Fitter, A.H. 2010. Substantial nitrogen acquisition by
arbuscular mycorrhizal fungi from organic material has implications for N
cycling. Proceedings of the National Academy of Sciences of the United
States of America 107:13754–13759. https://doi.org/10.1073/pnas.1005874107
Hodge, A.,
Storer, K. 2015. Arbuscular mycorrhiza and
nitrogen: Implications for individual plants through to ecosystems. Plant
Soil 386:1–19. https://doi.org/10.1007/s11104-014-2162-1
Hodge, A., Campbell, C.D., Fitter, A.H. 2001. An arbuscular mycorrhizal
fungus accelerates decomposition and acquires nitrogen directly from organic
material. Nature 413:297–299.
Horsch, C.C.A., Antunes, P.M., Kallenbach, C.M. 2023. Arbuscular
mycorrhizal fungal communities with contrasting life-history traits influence
host nutrient acquisition. Mycorrhiza 33:1-14. https://doi.org/10.1007/s00572-022-01098-x
Hou, E., Chen, C., Luo, Y., Zhou, G., Kuang, Y., Zhang, Y., Wen, D.
2018. Effects of climate on soil phosphorus cycle and availability in natural
terrestrial ecosystems. Global Change Biology 24(8): 3344–3356. https://doi.org/10.1111/gcb.14093
Jansa, J., Bukovská, P., Gryndler, M. 2013. Mycorrhizal hyphae as
ecological niche for highly specialized hypersymbionts or just soil
free-riders? Frontiers in Plant Science 4:1–8. https://doi.org/10.3389/fpls.2013.00134
Jeewani, P.H., Luo, Y., Yu, G., Fu, Y., He, X., Van Zwieten, L., Xu, J.
2021. Arbuscular mycorrhizal fungi and goethite promote carbon sequestration
via hyphal-aggregate mineral interactions. Soil Biology and
Biochemistry 162:108417. https://doi.org/10.1016/j.soilbio.2021.108417
Jiménez-Martínez, A., del
Carmen Gutiérrez-Castorena, M., Montaño, N.M., Gutiérrez-Castorena, E.V.,
Alarcón, A., Gavito, M.E. 2024. Micromorphology and thematic
micro-mapping reveal differences in the soil structuring traits of three
arbuscular mycorrhizal fungi. Pedobiologia 104:150953. https://doi.org/10.1016/j.pedobi.2024.150953
Johnson, N.C. 2010. Resource stoichiometry elucidates the structure and
function of arbuscular mycorrhizas across scales. New Phytologist
185:631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Jurado, E.,
Flores, J. 2005. Is seed dormancy under
environmental control or bound to plant traits? Journal of vegetation
Sciences 16:559–564. https://doi.org/10.1111/j.1654-1103.2005.tb02396.x
Kemmelmeier, K., Dos Santos, D.A., Grittz, G.S., Stürmer, S.L. 2022. Composition and seasonal variation of the arbuscular mycorrhizal
fungi spore community in litter, root mat, and soil from a subtropical rain
forest. Mycorrhiza 1–15. https://doi.org/10.1007/s00572-022-01084-3
Kemper,
W.D., Rosenau, R.C. 1986. Aggregate Stability and
Size Distribution. Methods of soil analysis. Part 1. In: Arnold K. (Ed.)
Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods, 2nd Ed.,
American Society of Agronomy/Soil Science Society of America, Madison, WI, USA,
pp. 425-444.
Klironomos,
J.N., Hart, M.M. 2002 Colonization of roots by
arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza
12:181–184. https://doi.org/10.1007/s00572-002-0169-6
Koide, R.T. 2000. Mycorrhizal symbiosis and plant reproduction. In:
Kapulnik, Y., Douds, D.D.J. (eds.), Arbuscular Mycorrhizas: physiology and
function, pp 19–46. Kluwer academic, Dordrecht. The Netherlands.
Koide, R.T.,
Kabir, Z. 2000. Extraradical hyphae of the
mycorrhizal fungus Glomus intrarradices can hydrolyse organic phosphate.
New Phytologist 148:511–517. https://doi.org/10.1046/j.1469-8137.2000.00776.x
Krüger, C., Kohout, P., Janoušková, M., Püschel, D., Frouz, J., Rydlová,
J. 2017. Plant communities rather than soil properties structure arbuscular
mycorrhizal fungal communities along primary succession on a mine spoil. Frontiers
in Microbiology 8:719. https://doi.org/10.3389/fmicb.2017.00719
Laiolo, P., Illera, J.C., Meléndez,
L., Segura, A., Obeso, J.R. 2015. Abiotic, biotic and
evolutionary control of the distribution of C and N isotopes in food webs. The
American Naturalist 185:169–182. http://dx.doi.org/10.5061/dryad.g4p92
Lehmann, A.,
Rillig, M.C. 2015. Understanding mechanisms of soil
biota involvement in soil aggregation: A way forward with saprobic fungi?. Soil
Biology and Biochemistry 88:298-302. https://doi.org/10.1016/j.soilbio.2015.06.006
Lehmann, A., Leifheit, E.F., Rillig, M.C. 2017. Mycorrhizas and soil
aggregation. In: Collins Johnson, N., Gehring, C., Jansa, J. (eds.), Mycorrhizal
mediation of soil, pp. 241-262. Elsevier.
Leigh, J., Fitter, A.H., Hodge, A. 2011. Growth and symbiotic
effectiveness of an arbuscular mycorrhizal fungus in organic matter in
competition with soil bacteria. FEMS Microbiology Ecology 76:428–438. https://doi.org/10.1111/j.1574-6941.2011.01066.x
Li, Y., Xie, Y., Liu, Z., Shi, L., Liu, X.,
Liang, M., Yu, S. 2023. Plant species identity and mycorrhizal type explain the
root-associated fungal pathogen community assembly of seedlings based on
functional traits in a subtropical forest. Frontiers in Plant Science 14:1251934.
https://doi.org/10.3389/fpls.2023.1251934
Ludwig-Müller, J. 2010. Hormonal responses in host plants triggered by arbuscular
mycorrhizal fungi. In: Kapulnik,
Y., Douds, D.D.J. (eds.), Arbuscular mycorrhizas: physiology and
function, pp. 169–190. Kluwer
academic, Dordrecht. The Netherlands.
Lugo, M.A., Ferrero, M., Menoyo, E.,
Estévez, M.C., Siñeriz, F., Antón, A. 2008. Arbuscular
mycorrhizal fungi and rhizospheric bacteria diversity along an altitudinal
gradient in South American Puna grassland. Microbial Ecology 55:705–713.
https://doi.org/10.1007/s00248-007-9313-3
Mayfield, M.M., Bonser, S.P., Morgan, J.W., Aubin, I., McNamara, S., Vesk,
P.A. 2010. What does species richness tell us about functional trait diversity?
Predictions and evidence for responses of species and functional trait
diversity to land‐use change. Global Ecology and Biogeography 19:423–431. https://doi.org/10.1111/j.1466-8238.2010.00532.x
Morris, E.K.,
Buscot, F., Herbst, C., Meiners, T., Obermaier, E., Wäschke, N.W., et al. 2013.
Land use and host neighbor identity effects on arbuscular mycorrhizal fungal
community composition in focal plant rhizosphere. Biodiversity and
conservation 22:2193-2205. https://doi.org/10.1007/s10531-013-0527-z
Nicolson, T.H. 1959. Mycorrhiza in the Gramineae. I. Vesicular-arbuscular
endophytes with special reference to the external phase. Transactions of the
British Mycological Society 42:421–438. https://doi.org/10.1016/S0007-1536(59)80043-7
Olivera-Morales, D.,
Castillo-Argüero, S., Guadarrama, P., Ramos-Zapata, J., Álvarez-Sánchez, J.,
Hernández-Cuevas, L. 2011. Establecimiento de plántulas de Quercus rugosa
Née inoculadas con hongos micorrizógenos arbusculares en un bosque templado de
México. Boletín de la Sociedad Botánica de México 89:115–121.
Paterson, E., Sim, A., Davidson,
J., Daniell, T.J. 2016. Arbuscular mycorrhizal hyphae promote
priming of native soil organic matter mineralization. Plant and Soil
408:243–254. https://doi.org/10.1007/s11104-016-2928-8
Perea-Rojas, Y.D.C., Arias,
R.M., Medel Ortiz, R., Trejo Aguilar, D., Heredia, G., Rodríguez Yon, Y. 2019. Effects of native arbuscular mycorrhizal and phosphate-solubilizing
fungi on coffee plants. Agroforestry Systems 93:961-972. https://doi.org/10.1007/s10457-018-0190-1
Piotrowski, J.S., Denich, T., Klironomos, J.N., Graham, J.M., Rillig, M.C.
2004. The effects of arbuscular mycorrhizas on soil aggregation depend on the
interaction between plant and fungal species. New Phytologist
164:365-373. https://doi.org/10.1111/j.1469-8137.2004.01181.x
Powell, J.R., Parrent, J.L., Hart, M.M., Klironomos, J.N., Rillig, M.C.,
Maherali, H. 2009. Phylogenetic trait conservatism and the evolution of
functional trade-offs in arbuscular mycorrhizal fungi. Proceedings of the
Royal Society Biological Sciences 276(1676):4237–4245. https://doi.org/10.1098/rspb.2009.1015
Pozo, M.J., Azcón-Aguilar, C.
2007. Unraveling mycorrhiza-induced resistance. Current
opinion in plant biology 10:393-398. https://doi.org/10.1016/j.pbi.2007.05.004
Pozo, M.J., Verhage, A.,
García-Andrade, J., García, J.M., Azcón-Aguilar, C. 2008. Priming
plant defence against pathogens by arbuscular mycorrhizal fungi. In:
Azcón-Aguilar, C., Barea, J.M., Gianinazzi, S., Gianinazzi-Pearson, V. (eds.), Mycorrhizas-functional
processes and ecological impact, pp. 123-135. Springer Berlin
Heidelberg. Berlin, Heidelberg.
Ramos-Zapata, J.,
Marrufo-Zapata, D., Guadarrama-Chávez, P., Solís-Rodríguez, U., Salinas-Peba,
L. 2013. Ruderal plants: temporary hosts of arbuscular
mycorrhizal fungi in traditional agricultural systems? Tropical and
Subtropical Agroecosystems 16:399–406.
Rillig, M.C.,
Mummey, D.L. 2006. Mycorrhizas and soil structure. New
phytologist 171:41-53. https://doi.org/10.1111/j.1469-8137.2006.01750.x
Roman-García, F.,
Yahuaca-Mendoza, M.P., Farias-Larios, J., López-Aguirre, J.G.,
Aguilar-Espinosa, S., del Rocío Flores-Bello, M. 2004. Hormonal
Concentration and Growth in Chili Plants Inoculated with Several Mycorrhizal
Fungus, Evaluated in Different Steps. HortScience 39:832–833. https://doi.org/10.21273/HORTSCI.39.4.832D
Sánchez-Fuente, G.J. 2019. Diversidad
funcional y disturbio crónico en el bosque húmedo de montaña de San Luis
Potosí, implicaciones para su conservación.
Schnoor, T.K., Lekberg, Y., Rosendahl, S., Olsson, P.A. 2011. Mechanical
soil disturbance as a determinant of arbuscular mycorrhizal fungal communities
in semi-natural grassland. Mycorrhiza 21:211–220. https://doi.org/10.1007/s00572-010-0325-3
Sepp, S.K., Davison, J., Jairus, T., Vasar, M., Moora, M., Zobel, M.,
Öpik, M. 2019. Non‐random association patterns in a plant–mycorrhizal fungal network
reveal host–symbiont specificity. Molecular ecology 28:365-378. https://doi.org/10.1111/mec.14924
Six, J., Elliott, E.T., Paustian, K. 2000. Soil macroaggregate turnover
and microaggregate formation: a mechanism for C sequestration under no-tillage
agriculture. Soil Biology and Biochemistry 32:2099-2103. https://doi.org/10.1016/S0038-0717(00)00179-6
Smith, S.E., Read, D.J. 2008. Mycorrhizal Symbiosis. 3rd
edition. San Diego, USA: Academic Press.
Smith, S.E.,
Smith, F.A. 2011. Roles of arbuscular mycorrhizas
in plant nutrition and growth: new paradigms from cellular to ecosystem scales.
Annual Review of Plant Biology 62:227–250. https://doi.org/10.1146/annurev-arplant-042110-103846
Solaiman, Z. 2024. Role of AMF in Organic Matter Decomposition, Carbon
Sequestration and Climate Change Mitigation. In: Parihar, M., Rakshit, A.,
Adholeya, A., Chen, Y (eds.), Arbuscular mycorrhizal fungi in sustainable
agriculture: Nutrient and crop management, pp. 131-141. Singapore: Springer
Nature Singapore.
Teste, F.P.,
Dickie, I.A. 2017. Mycorrhizas across successional
gradients. In: Collins Johnson, N., Gehring, C., Jansa, J. (eds.), Mycorrhizal
Mediation of Soil, pp. 67–89. Elsevier.
Tisserant, E., Malbreil, M., Kuo, A., Kohler, A., Symeonidi, A., Balestrini,
R., et al. 2013. Genome of an arbuscular mycorrhizal fungus provides insight
into the oldest plant symbiosis. Proceeding of the National Academy of
Sciences 110:20117–22. https://doi.org/10.1073/pnas.1313452110
Toljander, J.F., Lindahl, B.D., Paul, L.R., Elfstrand, M., Finlay, R.D. 2007.
Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth
and community structure. FEMS Microbiology Ecology 61:295–304.
https://doi.org/10.1111/j.1574-6941.2007.00337.x
van der Heyde, M., Ohsowski, B., Abbott, L.K., Hart, M. 2017. Arbuscular
mycorrhizal fungus responses to disturbance are context dependent. Mycorrhiza
27:431–440. https://doi.org/10.1007/s00572-016-0759-3
Varela-Fregoso, L., Mora-Velázquez, A., Chávez-Hernández, C.G., Martínez-Bernal,
A., García-Sánchez, R., Chimal-Sánchez, E., Montaño, N.M. 2017. Acaulospora
alpina y Ambispora fennica, dos registros nuevos de hongos
micorrizógenos arbusculares para México. Revista mexicana de biodiversidad
88:496-501. https://doi.org/10.1016/j.rmb.2017.06.005
Vázquez-Santos, Y. 2025. La
función de los hongos micorrizógenos arbusculares en especies de la vegetación
secundaria en un bosque templado (Doctoral dissertation). Facultad de
Ciencias, Universidad Nacional Autónoma de Mexico, México. 185 pp.
Vázquez-Santos, Y.,
Castillo-Argüero, S., Martínez-Orea, Y., Sánchez-Gallen, I., Vega-Frutis, R.,
Camargo-Ricalde, S.L., Hernández-Cuevas, L.V. 2019. The
reproductive phenology of Acaena elongata and its relation with
arbuscular mycorrhizal fungi. Symbiosis 79:129–140. https://doi.org/10.1007/s13199-019-00629-z
Vázquez-Santos, Y.,
Castillo-Argüero, S., Montaño, N.M., Espinosa-García, F.J., Flores-Ortiz, C.,
Martínez-Orea, Y. 2024. Arbuscular mycorrhizal fungi affect
early phenological stages of three secondary vegetation species in a temperate
forest. Plant Ecology 225:983-996. https://doi.org/10.1007/s11258-024-01448-z
Vega-Frutis,
R., Guevara, R. 2009. Different arbuscular
mycorrhizal interactions in male and female plants of wild Carica papaya
L. Plant and Soil 322:165-176. https//doi.org/10.1007/s11104-009-9903-6
Verbruggen,
E., Kiers, E.T. 2010. Evolutionary ecology of
mycorrhizal functional diversity in agricultural systems. Evolutionary
Applications 3:547-560. https://doi.org/10.1111/j.1752-4571.2010.00145.x
Veresoglou,
S. D., Rillig, M. C. 2012. Suppression of fungal
and nematode plant pathogens through arbuscular mycorrhizal fungi. Biology
letters 8:214-217. https://doi.org/10.1098/rsbl.2011.0874
Violi, H.A., Barrientos-Priego, A.F., Wright, S.F., Escamilla-Prado, E.,
Morton, J.B., Menge, J.A., Lovatt, C.J. 2008. Disturbance changes arbuscular
mycorrhizal fungal phenology and soil glomalin concentrations but not fungal
spore composition in montane rainforests in Veracruz and Chiapas, Mexico. Forest
Ecology and Management 254:276–290. https://doi.org/10.1016/j.foreco.2007.08.016
Vosátka, M.,
Gryndler, M. 2000. Response of micropropagated
potatoes to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Applied
Soil Ecology 15:145–152. https://doi.org/10.1016/S0929-1393(00)00090-1
Vosnjak, M., Likar, M., Osterc, G. 2021. The effect of mycorrhizal inoculum
and phosphorus treatment on growth and flowering of Ajania (Ajania pacifica
(Nakai) Bremer et Humphries) plant. Horticulturae 7:178. doi: https://doi.org/10.3390/horticulturae7070178
Wang, L., Tang, Z. 2022. How do arbuscular mycorrhizas affect reproductive
functional fitness of host plants?. Frontiers in Plant Science
13:975488. https://doi.org/10.3389/fpls.2022.975488
Wang, F., Zhang, L., Zhou, J., Rengel, Z., George, T.S., Feng, G. 2022.
Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: processes
and ecological functions. Plant and Soil 48:1-22. https://doi.org/10.1007/s11104-022-05621-z
Weber, S.E., Diez, J.M., Andrews, L.V., Goulden, M.L., Aronson, E.L.,
Allen, M.F. 2019. Responses of arbuscular mycorrhizal fungi to multiple
coinciding global change drivers. Fungal Ecology 40:62-71. https://doi.org/10.1016/j.funeco.2018.11.008
Welc, M., Ravnskov, S., Kieliszewska-Rokicka, B., Larsen, J. 2010.
Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal
fungi in root-free soil. Soil Biology & Biochemistry 42:1534–1540. https://doi.org/10.1016/j.soilbio.2010.05.024
Went, F.W.,
Stark, N. 1968. The biological and mechanical role
of soil fungi. Proceeding of the National Academy of Sciences
60:497–504. https://doi.org/10.1073/pnas.60.2.497
Xiao, D., Chen, M., He, X., Nie, Y., Jiang, N.,
Zhang, W., Wang, K. 2023. Soil nutrients and vegetation along a karst slope
gradient affect arbuscular mycorrhizal fungi colonization of roots rather than
bulk soil AMF diversity. Plant and Soil 489:139-154. https://doi.org/10.1007/s11104-023-06004-8
Zhang, J., Deng, L., Jiang, H., Peng, C., Huang,
C., Zhang, M., Zhang, X. 2021. The effects of elevated CO 2, elevated O 3,
elevated temperature, and drought on plant leaf gas exchanges: A global
meta-analysis of experimental studies. Environmental Science and Pollution
Research 28:15274-15289. https://doi.org/10.1007/s11356-020-11728-6
Zhang, M., Yang, M., Shi, Z., Gao, J., Wang, X. 2022. Biodiversity and
variations of arbuscular mycorrhizal fungi associated with roots along
elevations in Mt. Taibai of China. Diversity 14:626. https://doi.org/10.3390/d14080626
Zheng, Q., Hu, Y., Zhang, S., Noll, L., Böckle, T., Dietrich, M., Wanek,
W. 2019. Soil multifunctionality is affected by the soil environment and by
microbial community composition and diversity. Soil Biology and Biochemistry
136:107521. https://doi.org/10.1016/j.soilbio.2019.107521
Zhu, X., Song, F., Liu, F. 2017. Arbuscular mycorrhizal fungi and
tolerance of temperature stress in plants. In: Wu, Q-S. (ed.) Arbuscular
mycorrhizas and stress tolerance of plants, 163–194 pp. Springer,
Singapore.
Zhu, Y. G., Smith, S.E., Barritt, A.R., Smith, F.A. 2001. Phosphorus
(P) efficiencies and mycorrhizal responsiveness of old and modern wheat
cultivars. Plant and Soil 237:249-255.
Zobel, M. 2016. The species pool concept as a framework for studying
patterns of plant diversity. Journal of Vegetation Science 27:8-18. https://doi.org/10.1111/jvs.12333
Zobel, M., Öpik,
M. 2014. Plant and arbuscular mycorrhizal fungal
(AMF) communities–which drives which? Journal of Vegetation Science
25:1133–1140. https://doi.org/10.1111/jvs.12191
![]() , Silvia
Castillo-Argüero2
, Silvia
Castillo-Argüero2 ![]() , Arturo Jiménez-Martínez3
, Arturo Jiménez-Martínez3 ![]()



