Intermittent water deficit in
Mediterranean freshwater ecosystems
Water scarcity, the loss of freshwater ecosystems,
and the occurrence of drought are among the most urgent environmental
challenges of the 21st century (Collins et al. 2009). With the growing
global population and the impacts of climate change, including shifts in
rainfall patterns, the already stressed freshwater ecosystems face further
threats. Drought, a recurring and damaging natural hazard, remains one of the
least understood phenomena (Gornall
et al. 2010; Lesk et al. 2016). Unlike aridity, which
characterizes permanently low rainfall areas, drought can occur in regions with
varying levels of rainfall (Wilhite
2000). However, both the frequency and intensity of
droughts are expected to increase worldwide due to global climate change and
rising demands for freshwater resources (Naylor and Coleman-Derr 2018).
In Europe, meteorological and hydrological
droughts have become more severe and frequent, particularly in southwestern and
central regions. Southern Europe, in particular, is projected to experience the
greatest increase in drought conditions, leading to intensified competition
among various water users, such as agriculture, industry, tourism, and
households (EPA www.epa.gov). Arid and semi-arid climate areas are particularly vulnerable to
drought, posing a significant risk to the integrity and functioning of river
networks (Bonada and Resh
2013; Prudhomme et al. 2014). In southern
Europe, Mediterranean intermittent rivers are heavily impacted by climate
change and human water demands, resulting in water scarcity that pushes these
ecosystems closer to resembling terrestrial systems (Datry et al. 2017). By definition, Intermittent rivers and Ephemeral Streams (IRES)
are river water bodies characterized by temporary flow. Up to date, researchers
worldwide are investigating the consequences of drought and the functioning of
intermittent freshwater ecosystems to assess the extent to which they are
transitioning into terrestrial-like systems. While the historical repetition of
wet and dry events in intermittent rivers may promote biota and microbiota
adaptation, prolonged desiccation periods can lead to unforeseen consequences.
Relevance of habitat patchiness for
streambed microbiota
The temporal instability of flow conditions
in streams plays a crucial role in shaping the habitat patchiness within the
streambed, both longitudinally, vertically, and laterally, driven by
hydrological, climatic, and geomorphological factors (Steward et al. 2012). The reduction in surface water during intermittent flow creates a
mosaic of habitats and microhabitats that can support diverse aquatic and
terrestrial biota, including microbiota (Costigan et al. 2016). The dynamic
nature of intermittent streambeds and their boundaries, constantly changing as
the stream contracts or expands, allows for the occupation of distinct
communities in different spots within the streambed, increasing overall
biodiversity and compensating for the decrease observed during no-flow
conditions (Larned et al.
2010). This intimate link between streams and the
surrounding terrestrial environment is reflected in the streambed compartment.
During the desiccation phase, in addition
to isolated moist zones and dry bare ground spots, the intermittent streambed
often contains specific habitats that better retain moisture, such as leaf
litter packs (on the surface or buried within the sediment), woody debris, and
algal mats (Steward et al.
2012; Romaní et al. 2017). The mosaic of
habitats created under fluctuating stream flows, particularly during
desiccation, is of significant importance as it provides refuge for the
streambed microbiota, including prokaryotes and eukaryotes, inhabiting the
sediment. These refuges enable microbes to withstand harsh conditions
characterized by water scarcity, direct radiation, limited nutrient diffusion,
and reduced availability of organic matter. Given the pivotal role of streambed
microbiota in driving key ecosystem functions, these streambed habitats ensure
the maintenance of microbial-mediated stream processes such as nutrient and
carbon cycling (Romaní et al.
2017).
Importantly, the highly variable nature of
microhabitats within the streambed, shaped by the intermittent hydrological
cycle, can lead to patchy distributions of microbial taxa and changes in
ecosystem functions over space and time, creating waves of microbial diversity
and influencing overall system functioning (Datry et al. 2017; Vadher et al. 2017). The intermittent streambed ecotone harbors unique microbial
diversity, including assemblages that are more or less adapted to hydrological
fluctuations and play essential roles in biogeochemical processes within the
stream (Barthès et al. 2015; Logue et al. 2016). As the streambed transitions from wet to extremely dry
conditions, it acquires similar features to nearby soils, highlighting the
adaptability of streambed microbiota to changing hydrological conditions (Morandi et al. 2014; Arce et al. 2019).
The interface between aquatic and
terrestrial ecosystems
The extent to which dry streambeds resemble
soils is closely associated with the duration of the dry phase and the
frequency of rewetting episodes (Harms and Grimm 2012; Mori et al. 2017), along with various environmental factors such as solar radiation,
sediment water retention capacity, and shadow cover, which influence the
preservation of aquatic characteristics within the streambed. Dry streambeds
exhibit numerous similarities to soil systems, particularly during prolonged
periods of dryness and infrequent inundation events, which contribute to the
acquisition of soil-like features (Arce et al. 2019).
Recent studies on soil systems have
revealed that microbiota generally adapt well to the initial desiccation
process, as certain bacterial and fungal species exhibit drought tolerance and
exhibit low rates of activity and growth during water scarcity (Schimel et al. 2010). However, when desiccation periods become prolonged, biological
activities become energetically costly, and microorganisms experience direct
physiological stress due to water restriction and limited resources.
Interestingly, prolonged desiccation may also lead to reduced predation
pressure on microbes (Görres
et al. 1999), as protozoa require water-filled
pores for foraging (Stefan et
al. 2014). Desiccation stress further enhances the
similarities between aquatic and terrestrial microbial communities, driven by
dispersal and colonization from adjacent terrestrial systems or through the
adaptation and selection of taxa capable of thriving in specific dry-habitat
conditions (Febria et al.
2015; Monard et al. 2016; Sabater et al. 2016). For instance, the presence of a higher abundance of Gram-positive
bacterial species or the survival of fungal species with amphibian-like
characteristics in intermittent aquatic systems could reflect the initial
stages of a terrestrial system originating from alluvial deposits (Morandi et al. 2014; Mori et al. 2017; Arce et al. 2019).
Coupled and uncoupled functional and
structural microbial responses to desiccation
In today's context, the increasing
frequency of desiccation and rewetting episodes, as well as sporadic storms
interrupting prolonged dry periods, pose a threat to microbial biodiversity and
functioning (Findlay 2010). Changes in microbial functions under hydrological constraints can
either be coupled or uncoupled with shifts in microbial community structure,
such as diversity and composition (e.g., Marxsen et al. 2010; Manzoni et al. 2012). The relationship between microbial community structure and
function remains a subject of debate in microbial ecology, and studies
investigating the function-structure relationships of microbial communities in
intermittent rivers are still limited. Recent research has reported conflicting
findings regarding the ability of microbial communities inhabiting intermittent
streambeds to maintain their functions and structure during wet and dry cycles
(Barthès et al. 2015; Febria et al.
2015).
Microorganisms demonstrate great resilience
and resistance to (repeated) disturbances, such as hydrological changes,
indicating their capacity to remain relatively unchanged and revealing weakly
coupled or uncoupled patterns between community function and structure (Frossard et al. 2012; Gibbons et al.
2014). The response of microbial communities to
drought can exhibit functional plasticity, where the composition remains stable
while functions change, or functional redundancy, where the composition varies
but functions remain consistent (Allison and Martiny 2008). However,
microbial functional stability is often compromised compared to structural
stability under different hydrological conditions. The alternation between wet
and dry phases can affect the capabilities of microbial heterotrophs to degrade
organic matter through the utilization of different extracellular enzymes. This
can result in the conservation of overall community composition, indicating
functional plasticity (Romaní
et al. 2013; Freixa et al. 2016; Louca et al. 2018). Conversely, other studies have observed differences in community
composition or diversity while functions remain similar, suggesting a high
degree of functional redundancy (Frossard et al. 2012; Lear et al. 2014; Wagner et al.
2014).
The collective resistance (ability to cope
with stress) and resilience (ability to recover quickly) of microbial
communities in response to drought disturbances play a crucial role in
maintaining community stability under varying intensities of disturbances (Allison and Martiny 2008). Although several studies have explored the dynamics of microbial
communities in intermittent streams in response to desiccation, the threshold
beyond which microbial communities would be completely altered, both in terms
of structure and function, by prolonged desiccation or rewetting, remains
unclear. This knowledge gap motivated the focus of this revision manuscript.
In the following subsections we present a
compilation of microbial functional and structural responses from two distinct
studies conducted in different settings: a laboratory experiment using
microcosms to simulate long-term desiccation (> 5 months; Gionchetta et al. 2019) and a field study conducted across multiple sites, encompassing a
wide range of intermittency (including dry periods of up to 8 months; Gionchetta et al. 2020). This combination of experimental and field approaches allowed us
to examine the effects of desiccation under controlled conditions as well as in
natural hydrological settings. The laboratory experiment provided us with the
ability to control external factors and isolate the specific impact of
desiccation, while the field study allowed us to capture the complexity of
natural hydrological variations. By integrating and comparing the findings from
both studies, we uncovered important insights into the responses of microbial
communities to desiccation.
Functional responses: organic matter degradation capabilities
As a key functional response linked to the
streambed biofilm role in biogeochemical cycling (Battin et al. 2016), the organic matter degradation capabilities were analyzed by
means of the extracellular enzyme microbial activities in streambed sediments.
Specifically, we testes the microbial capacity to degrade simple
polysaccharides, such as the hemicellulose and cellobiose degradation activity
(β-xylosidase (XYL); β-Glucosidase,
GLU) and the abiilty to degrade lignin compounds (phenol-oxidase (PHE)
activity). Disparities in organic matter quality and quantity between the
different hydrological phases may
translate into differences in organic matter decomposition capabilities (Ylla et al. 2010). A drying streambed may accumulate greater amount of organic
matter (e.g., from animal or vegetal
debris) compared with other hydrological
states, especially in arid, semiarid, and Mediterranean regions where the vegetation is limited to the riparian zones (Steward et al. 2012; Arce et al. 2019). Even under dry conditions, extracellular enzyme activities have
been measured and related to the microbial organic matter decomposition in
stream sediments (Marxsen et al. 2010; Zoppini et al.
2014). Previous studies also linked the type of enzyme activities activation to the distinct
hydrological phases (drought
vs flooding events) in intermittent
rivers (Ylla et al. 2010; Romaní et al.
2013; Freixa et al. 2016). Here, our results
show differing uses of enzymatic activities depending on the hydrological phase
and streambed condition (i.e., flowing water, fragmentation-
including intermittent sites and pool condition-, and dry in Fig. 1).
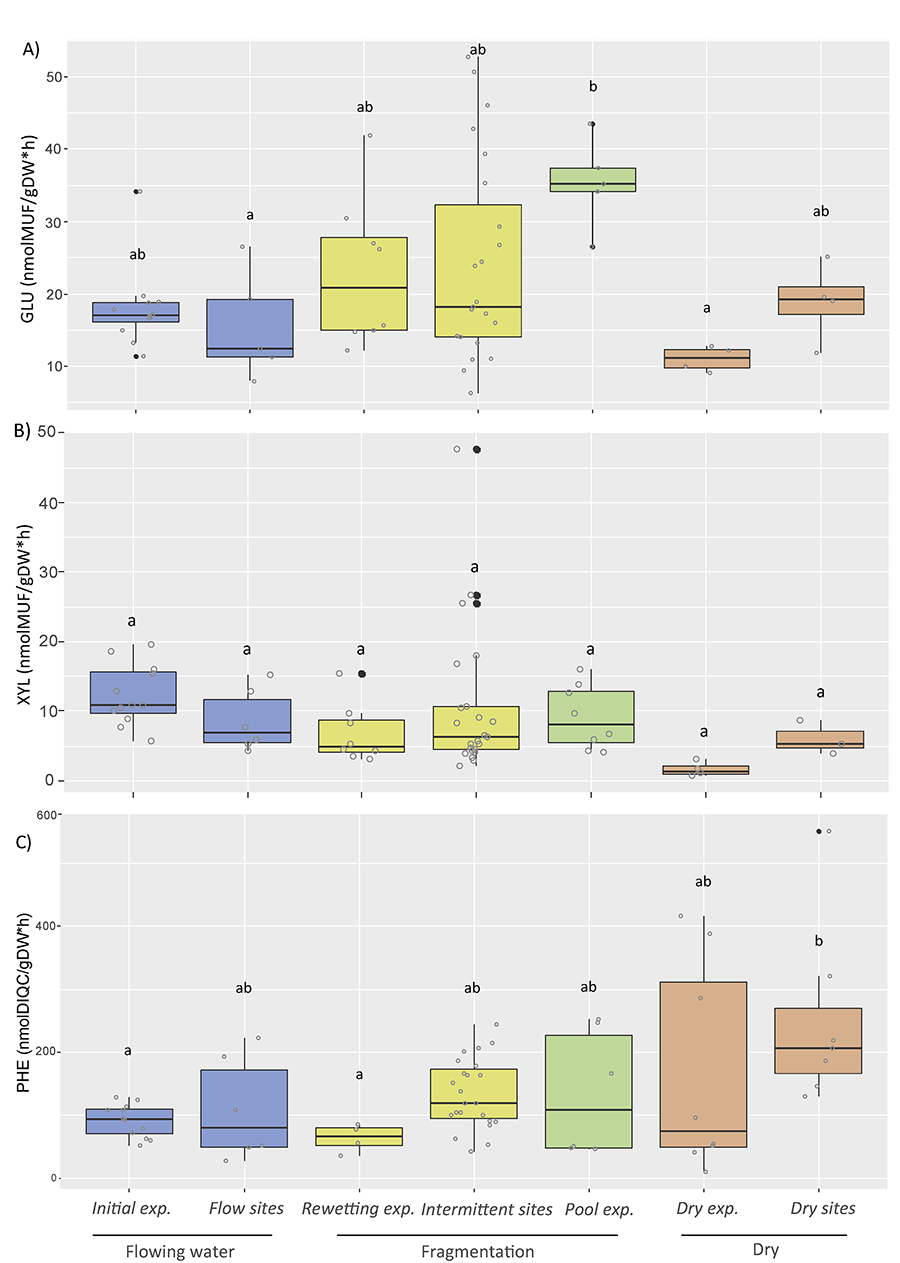
Figure 1. Box plots of extracellular enzyme activities from streambed
sediments: A) β-Glucosidase, GLU, B) β-Xylosidase, XYL and C)
Phenol-oxidase, PHE. Data included correspond to laboratory and field studies
from Gionchetta et
al. (2019 and 2020), representing different hydrological phases such as Flowing waters,
Fragmentation and Dry conditions. Laboratory experimental (exp.) data
correspond to the initial streambed sediment incubated from a permanent
Mediterranean stream (called Initial exp., n=3), the streambed sediment
submitted to desiccation (150 days) interrupted by a storm event at day 90 and
sampled just after the storm (called Rewetting exp., n=3), the streambed
sediment water saturated for 150 days, simulating a pool (called Pool exp., n=3),
and the streambed sediment desiccated for 150 days (called Dry exp., n=3) (Gionchetta et al. 2019). Field data correspond to streambed sites where hydrology was
monitored for the previous 8 months before sampling and included permanent
streams (called Flow sites, n= 5), intermittent streams (dry <30% of the
time, called Intermittent sites, n=21), and long-dry streams (dry >85% of
the time, ca. more than 5 months, called Dry sites n=6) (Gionchetta et al. 2020).
Figura 1. Diagramas de caja de las actividades
enzimáticas extracelulares de los sedimentos de los ríos: A)
β-Glucosidasa, GLU, B) β-Xilosidasa, XYL y C) Fenol-oxidasa, PHE.
Los datos incluidos corresponden a estudios de laboratorio y campo de Gionchetta et al. (2019 y 2020), representando diferentes fases
hidrológicas como Aguas fluyentes, Fragmentación y Condiciones secas. Los datos
experimentales (exp.) de laboratorio corresponden al sedimento inicial del
lecho del arroyo incubado de un arroyo mediterráneo permanente (llamado exp.
inicial, n=3), el sedimento del lecho del arroyo sometido a desecación (150
días) interrumpida por un evento de tormenta en el día 90 y muestreado justo
después de la tormenta (llamado exp. de rehumectación, n=3), el sedimento del
lecho del arroyo saturado de agua durante 150 días, simulando una charca
(llamado Pool exp., n=3), y el sedimento del lecho del arroyo desecado durante
150 días (llamado Dry exp., n=3) (Gionchetta et
al. 2019). Los datos de campo corresponden a sitios del lecho del arroyo
donde la hidrología fue monitoreada durante los 8 meses previos al muestreo e
incluyeron ríos permanentes (llamados sitios de Flujo, n= 5), ríos
intermitentes (secos <30% del tiempo, llamados sitios Intermitentes, n=21),
y ríos de secado prolongado (secos >85% del tiempo, ca. más de 5 meses,
llamados sitios Secos n=6) (Gionchetta et al.
2020).
The capacity to degrade simple
polysaccharides, such as the cellobiose degradation activity (β-Glucosidase, GLU) showed high variability and there
was a no clear inhibition of this activity due to increasing drought (i.e.,
from flowing water sediments to long-term dry sediments, Fig. 1A), as similarly observed in previous studies (Timoner et al. 2012; Freixa et al.
2016).
However, GLU activity tended to be higher
in fragmentation phases and variability was greatest in intermittent
conditions, such as in streambeds from intermittent sites (being dried between
10 and 30% of the previous 8 months before sampling) and in experimental
samples corresponding to sediment submitted to a storm after 90 days of drought
(intermittent sites and rewetting exp. in Fig. 1). These results underline
the importance of the wet events moistening the dry sediment that may occur in intermittent
sites, which may enhance the release of labile compounds from accumulated
organic matter during previous drought; this mobilization might be highly
variable between sites (Evans et al. 2014; Shumilova et
al. 2019). Similarly, increases in respiration
activity have been observed in intermittent streambeds especially in rewetting
episodes and related to the fast use of accumulated organic matter (Coulson et al. 2022). Furthermore, in the water saturated condition of the experimental
approach simulating a pool (i.e., Pool exp. in Fig. 1A),
the significant peaks of GLU activity
have been associated to the algal colonization, typically
occurring in these microhabitats, therefore considered
as a microbial refuge under harsh conditions (Larned et al. 2010; Casas-Ruiz et al. 2016).
This suggest that during fragmentation, degradation of simple polysaccharides
may be enhanced by the combination of activity at the different habitat
patches.
Under prolonged desiccation periods the
results from β-xylosidase (XYL) and phenol-oxidase (PHE) activities performed
opposite patterns (e.g. reduction of XYL and increase of PHE), for both experimental and field studies
(Figs. 1B, 1C). In the case of XYL, the slight reduction observed under extreme
dry condition (Fig. 1B), was related to the
potential change of the quality of the organic matter stored in the surface sediment during the desiccation phase (Ylla et al. 2010; Romaní et al.
2013) and may indicate a decrease on the
degradation of simple polysaccharides from hemicellulose origin (typically
being part of plant material). This may indicate that these first degradable
hemicellulose polysaccharides from plant material have been already decomposed
in previous wet phases. This pattern was especially
clear for the laboratory experiment (i.e., Dry exp. Fig. 1B)
whereas in the fieldwork the tendency
was weaker. Contrarily, PHE activity
resulted in a significantly increase
when increasing dryness conditions in the streambed (Fig. 1C). In the dried streambeds, the accumulation of allochthonous debris mostly composed
by recalcitrant materials
would enhance degradation of lignin-like materials (Sinsabaugh 2010; Burns et al. 2013). Importantly, the XYL and PHE enzyme activities were the most
responsive enzymes to the desiccation experiment (Gionchetta et al. 2019) and to some extent to the natural intermittency gradient (Gionchetta et al. 2020). Bearing in mind the
complexity and the multiple factors that could influence the microbial
functional, the extracellular enzymes
could be considered as useful microbial
functional markers for the detection
of extreme drying conditions, behind which the resilience
of the microbial-mediated ecosystem functions could be compromised. In
particular, the greater utilization
of PHE among the intermittent streambeds, submitted to long-term drought, could be used as a reference for
potential begin of transition from freshwater to terrestrial systems.
Microbial
community diversity and taxonomic composition responses
The hydrological fluctuations from flowing to extremely dry conditions fragment
the intermittent river path
creating microhabitats, recognized as microbial refuges (Romaní et al.
2017). The stream path fragmentation and the
variation of the quality and quantity
of organic matter, together with changes in water availability, can change the
streambed microbial diversity and composition
(Marxsen et al. 2010; Freixa et al.
2016). Changes in streambed community composition could be coupled or not to functional changes (as those described above). The
comparison between the streambed bacterial diversity
(measured as Shannon and Richness diversity indices)
obtained from the experimental and field studies tended to decrease in
the driest conditions (Fig. 2). In
spite of the large variability observed among the results obtained, significant diversity reductions have been observed
after long dry period, especially from the laboratory results (Fig. 2).
In terms of bacterial taxonomic
composition, changes in the most abundant classes colonizing the streambed were observed during the distinct
hydrological conditions in both laboratory and field studies (Fig. 3) and
a transition from Gram-negative to Gram-positive bacteria. The experimental and
field data comparison showed the transition of some taxa abundance, differentiating between two main types. The first type consisted in those that tended
to reduce their abundances when increasing drought
duration (e.g. Beta- and Delta-Proteobacteria and Bacteroiidia,
Fig. 3) which could be defined as “drought
sensitive”; the second type included those taxa that increased their abundances
under long-drought (e.g. Actinobacteria, Bacilli and Thermoleophilia
Fig. 3) which could be defined as “drought-adapted”. Other taxa
that were not significantly modifying their abundances (e.g. Bacteroidetes and Alpha-
and Gamma-Proteobacteria) could be
defined as “drought resistant”. These patterns were similar for both laboratory
and field experiments. As previously
observed from different studies on soil and
streambed microbial communities, hydrological changes could influence the taxa
selection (e.g. abundance of
specific classes) able to cope with osmotic stress (Schimel et al. 2007; Romaní et al.
2013; Zoppini et al. 2014). The
“drought-adapted” classes observed in
our studies have been already identified as able to cope with desiccation
periods in previous research
mainly focused on soil microbial
composition changes under drought conditions (Schimel et al. 2007; Manzoni et al.
2012; Barnard et al. 2013, 2014; Meisner et al. 2018; Naylor
and Coleman-Derr 2018). Furthermore, recent research reported
similar adaptation of bacteria
and fungi inhabiting soils and dry streambed submitted to frequent and intense dry conditions, such as
Gram-positive bacterial cells and fungi with thicker cell walls that help to
better cope with the osmotic stress (Jones and Lennon 2010; Yuste et al. 2011; Manzoni et al. 2012; Zeglin 2015).
All of these evidences strengthened the
similarity between the dry streambed systems studied and a (dry)-soil environment, suggesting that prolonged and
unusual dry periods could boost the terrestrial transition of the aquatic intermittent ecosystem.
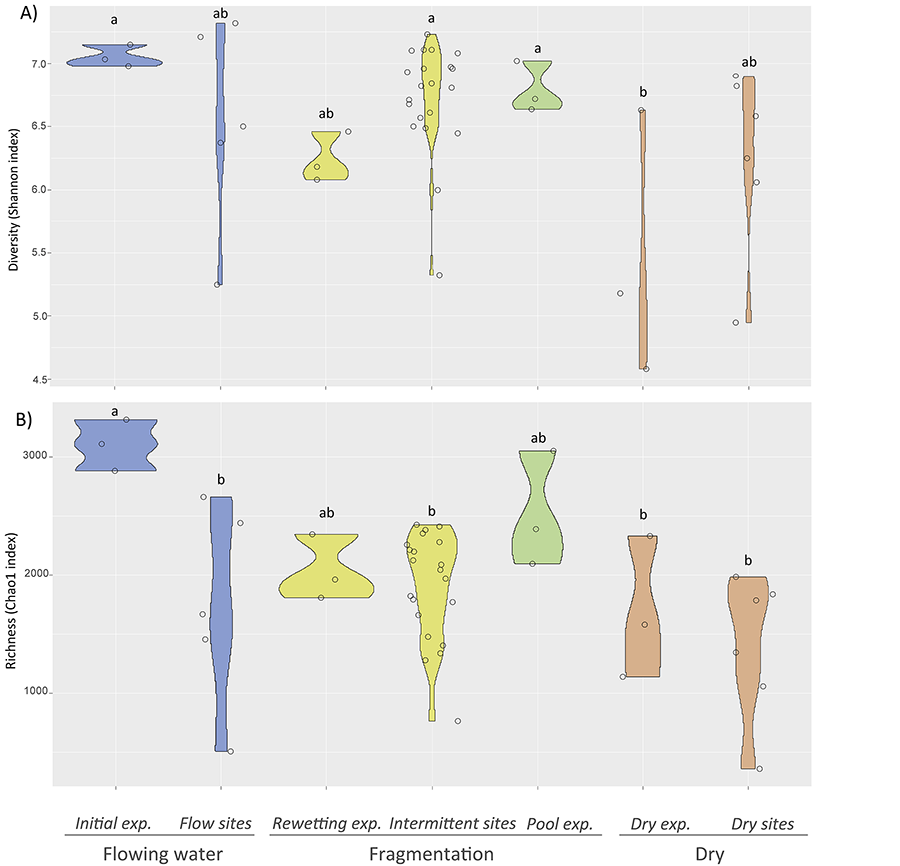
Figure 2. Box plots of
bacterial diversity indices from streambed sediments: A) Shannon-Wiener
diversity index B) Richness Chao1
diversity index. Data included correspond to laboratory and field studies from Gionchetta et al. (2019 and 2020), representing
different hydrological phases such as Flowing waters, Fragmentation and Dry
conditions. Laboratory experimental (exp.) data correspond to the initial
streambed sediment incubated from a permanent Mediterranean stream (called
Initial exp., n=3), the streambed sediment submitted to desiccation (150 days)
interrupted by a storm event at day 90 and sampled just after the storm (called
Rewetting exp., n=3), the streambed sediment water saturated for 150 days,
simulating a pool (called Pool exp., n=3), and the streambed sediment
desiccated for 150 days (called Dry exp., n=3) (Gionchetta
et al. 2019). Field data correspond to streambed sites where
hydrology was monitored for the previous 8 months before sampling and included
permanent streams (called Flow sites, n= 5), intermittent streams (dry <30%
of the time, called Intermittent sites, n=21), and long-dry streams (dry
>85% of the time, ca. more than 5 months, called Dry sites n=6) (Gionchetta et al. 2020). The
letters indicate significant differences between the categories tested
with one-way ANOVA and Tukey test.
Figura 2. Diagramas de
cajas de los índices de diversidad bacteriana de los sedimentos de los ríos: A)
Índice de diversidad de Shannon-Wiener B) Índice de diversidad Richness
Chao1. Los datos incluidos corresponden a estudios de laboratorio y de campo de
Gionchetta
et al. (2019 y 2020), representando diferentes fases hidrológicas como Aguas fluyentes,
Fragmentación y Condiciones secas. Los datos experimentales (exp.) de
laboratorio corresponden al sedimento inicial del lecho del arroyo incubado de
un arroyo mediterráneo permanente (llamado exp. inicial, n=3), el sedimento del
lecho del arroyo sometido a desecación (150 días) interrumpida por un evento de
tormenta en el día 90 y muestreado justo después de la tormenta (llamado exp.
de rehumectación, n=3), el sedimento del lecho del arroyo saturado de agua
durante 150 días, simulando una charca (llamado Pool exp., n=3), y el sedimento
del lecho del arroyo desecado durante 150 días (llamado Dry exp., n=3) (Gionchetta et al.
2019). Los datos de campo
corresponden a sitios del lecho del arroyo donde la hidrología fue monitoreada
durante los 8 meses previos al muestreo e incluyeron ríos permanentes (llamados
sitios de Flujo, n= 5), ríos intermitentes (secos <30% del tiempo, llamados
sitios Intermitentes, n=21), y ríos de secado prolongado (secos >85% del
tiempo, ca. más de 5 meses, llamados sitios Secos n=6) (Gionchetta et al.
2020). Las letras indican
diferencias significativas entre las categorías probadas con ANOVA
unidireccional y test de Tukey.
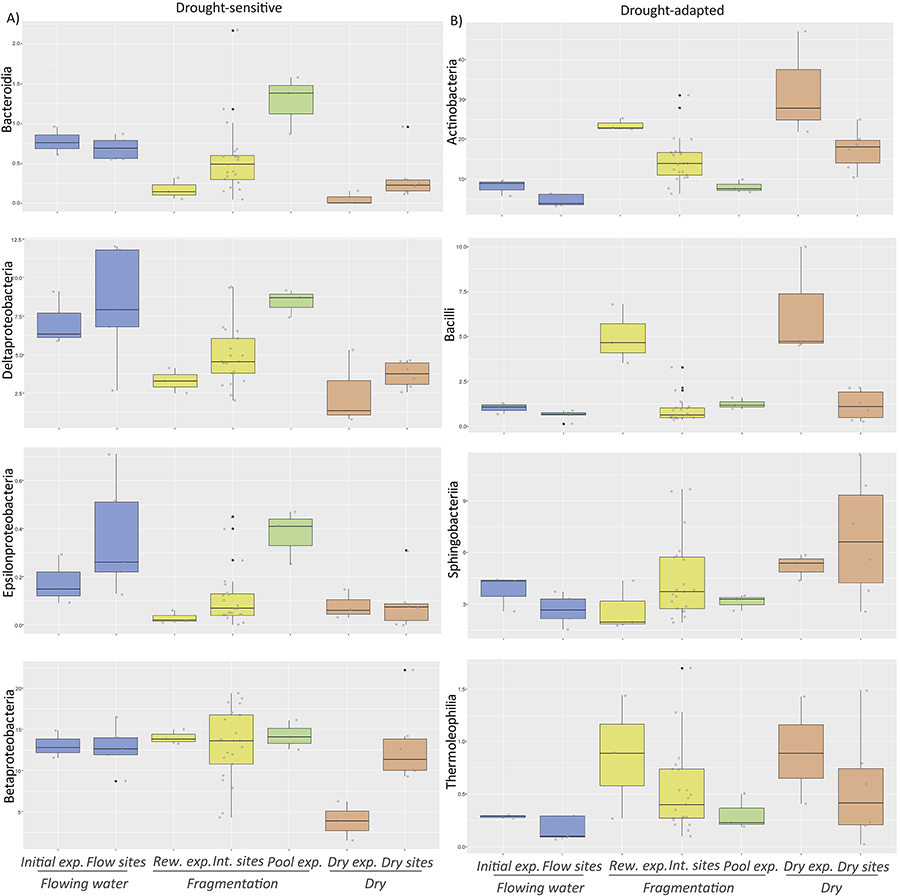
Figure 3.
Box plots of the 13 most abundant bacterial taxa
found in the streambeds from both the experiment and field studies: A)
Drought-sensitive (decreasing relative
abundance when increasing drought length), B) Drought-adapted (increasing relative abundance
when increasing drought
length). Data included
correspond to laboratory and field studies from Gionchetta et al. (2019 and 2020), representing different hydrological phases such as Flowing
waters, Fragmentation and Dry conditions. Laboratory experimental (exp.) data
correspond to the initial streambed sediment incubated from a permanent
Mediterranean stream (called Initial exp., n=3), the streambed sediment
submitted to desiccation (150 days) interrupted by a storm event at day 90 and
sampled just after the storm (called Rewetting exp., n=3), the streambed
sediment water saturated for 150 days, simulating a pool (called Pool exp., n=3),
and the streambed sediment desiccated for 150 days (called Dry exp., n=3) (Gionchetta et al. 2019). Field data correspond to streambed sites where hydrology was
monitored for the previous 8 months before sampling and included permanent
streams (called Flow sites, n= 5), intermittent streams (dry <30% of the
time, called Intermittent sites, n=21), and long-dry streams (dry >85% of
the time, ca. more than 5 months, called Dry sites n=6) (Gionchetta et al. 2020).
Figura 3. Diagramas de cajas de los 13 taxones
bacterianos más abundantes encontrados en los lechos de los ríos tanto en el
experimento como en los estudios de campo: A) Sensibles a la sequía
(abundancia relativa decreciente al aumentar la duración de la sequía), B)
Adaptados a la sequía (abundancia relativa creciente al aumentar la duración de
la sequía). Los datos incluidos corresponden a los estudios de laboratorio y
campo de Gionchetta et al. (2019 y 2020), representando diferentes fases
hidrológicas como Aguas fluyentes, Fragmentación y Condiciones secas. Los datos
experimentales (exp.) de laboratorio corresponden al sedimento inicial del
lecho del arroyo incubado de un arroyo mediterráneo permanente (llamado exp.
inicial, n=3), el sedimento del lecho del arroyo sometido a desecación (150
días) interrumpida por un evento de tormenta al día 90 y muestreado justo
después de la tormenta (llamado exp. de rehumectación, n=3), el sedimento del
lecho del arroyo saturado de agua durante 150 días, simulando una charca
(llamado Pool exp., n=3), y el sedimento del lecho del arroyo desecado durante
150 días (llamado Dry exp., n=3) (Gionchetta et
al. 2019). Los datos de campo corresponden a sitios del lecho del arroyo
donde la hidrología fue monitoreada durante los 8 meses previos al muestreo e
incluyeron ríos permanentes (llamados sitios de Flujo, n= 5), ríos
intermitentes (secos <30% del tiempo, llamados sitios Intermitentes, n=21),
y ríos de secado prolongado (secos >85% del tiempo, ca. más de 5 meses,
llamados sitios Secos n=6) (Gionchetta et al.
2020).
Integrating field and laboratory
experiments to reveal potential microbial indicators of desiccation
The combination of both experimental and
field data provided valuable insights into the advantages and limitations of
each approach. Laboratory experiments offer a higher degree of experimental
control, allowing for replicability and the ability to simulate specific
conditions of streambeds undergoing long desiccation periods. By using columns
of stream sediment, we were able to simplify the experimental conditions,
facilitating the understanding of ecological patterns and processes (Jessup et al. 2004). However, it is important to acknowledge the disadvantages of
laboratory approaches, such as the potential oversimplification and reduction
of spatial and temporal scales. These limitations restrict the direct
extrapolation and interpretation of experimental results to natural aquatic
ecosystems, and caution should be exercised when making such inferences.
On the other hand, field observations
provide a more realistic representation of the natural complexity of
intermittently dried streambeds. However, the inherent complexity also presents
challenges in identifying and obtaining clear patterns and conclusions. Proper
data analysis techniques, such as multiple factor modeling or comparative
approaches, are crucial for dealing with the large environmental variability
encountered in the field.
The integrated comparison between the
laboratory experiments and field observations allowed for a more accurate
description of the complex microbial dynamics that exist within intermittently
dried streambeds (Fig. 4). By
leveraging the strengths of both approaches, we were able to gain a
comprehensive understanding of the microbial responses to desiccation,
incorporating both controlled experimental conditions and the ecological
complexity of natural systems. This integrated approach enhances our ability to
elucidate the underlying mechanisms and ecological implications of microbial
dynamics in intermittently dried streambeds.
Altogether,
several tendencies converge in both approaches and are those we propose as
endpoints of desiccation. One notable finding was the functional shift observed
in the utilization of organic matter, indicating an adaptive response to the
extremely dry conditions, with the clearest response being the increased lignin
degradation capacity (PHE activity) respect to degradation of simple
polysaccharides (GLU & XYL activities). A further functional response was
the great variability and enhanced capacity to degrade simple polysaccharides
(GLU activity) in intermittency conditions usually taking place during flowing
fragmentation phases. Additionally, we observed a tendency of decreasing
bacterial diversity in the dry conditions together with a change in the
relative abundance of certain microbial taxa and a general shift from
Gram-negative to Gram-positive bacteria (Fig. 4).
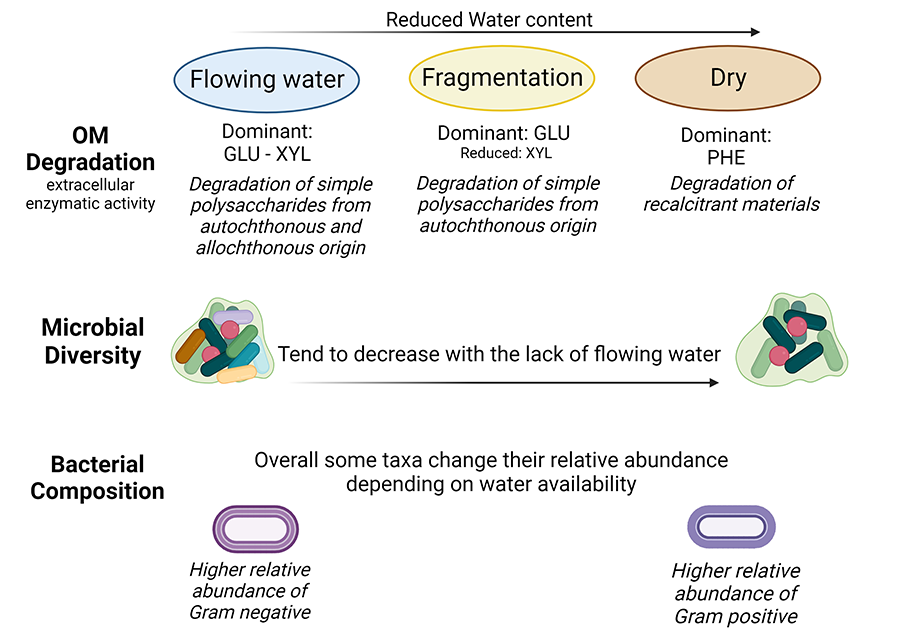
Figure 4. Diagram illustrating the main findings
obtained from this data
comparison. The acronyms
GLU, XYL and PHE indicate
the three extracellular enzyme activities: β- Glucosidase, β-Xylosidase and Phenol-oxidase, respectively. Flowing water, Fragmentation and Dry indicate
three distinct status of the streambed. Microbial diversity
indicates the measures of Shannon-Wiener and Richness indices.
Figura 4. Diagrama que ilustra los principales
resultados obtenidos de esta comparación de datos. Las siglas GLU, XYL y PHE
indican las tres actividades enzimáticas extracelulares: β- Glucosidasa,
β-Xilosidasa y Fenol-oxidasa, respectivamente. Agua fluyente, Fragmentación y
Seco indican tres estados distintos del lecho del arroyo. La diversidad
microbiana indica las medidas de los índices de Shannon-Wiener y de riqueza.
Above these promising tools, we need to be
aware of the limitations of the provided conclusions from the reported studies.
One notable limitation is the absence of a comprehensive investigation into
certain communities, such as fungi and protozoa, which play a crucial role in
the overall response of streambed microbial communities to drought stress.
Understanding the dynamics of the entire microbiota in the face of
environmental changes necessitates studying these groups. In addition to
examining the individual responses of fungi and protozoa during prolonged dry
periods, there is a lack of knowledge regarding the interconnections within the
microbial food web (Weitere
et al. 2018). Future studies should aim to address
this gap in the literature, as it would help refine our understanding and
ascertain the universality of microbiome trends under consistent drought
stress. Specifically, investigating the extent to which streambed microbial
composition is modulated between different groups and how these relationships
differ under drought conditions would be valuable. An additional hindrance to
analyzing the microbiota of dry streambeds is the methodology employed to
assess the impact on associated communities. The integration of molecular and
functional tools, which serve as proxies for ecosystem processes, has become
crucial for next-generation studies. Employing a multifactorial modeling
approach is considered the initial step towards effectively analyzing the
changing environment. Therefore, future studies should combine laboratory and
field approaches to ensure more meaningful and reliable conclusions.
Undoubtedly, the temporal and spatial
scales are also critical factors when comparing microbial responses across
different stream sites. Future research should incorporate larger time and
space series, coupled with specific characterization of the stream sites at
both habitat and catchment scales. This comprehensive approach would enable
better modeling of the potential effects resulting from increased drought
conditions. Furthermore, future studies should investigate the impact of
agricultural activities on riparian vegetation and its link to the desiccation
period. Revealing potential additive negative effects, such as reduced
microbial diversity and functions (Griffith et al. 2019), can shed light on
the consequences of agricultural practices on streambed ecosystems.
In conclusion, these suggestions serve as a
foundation for future investigations and can help mitigate the impacts of
global change on streambed microbiota functions, ultimately safeguarding
freshwater ecosystems and ensuring water security.
Contribution of the authors
GG:
Conceptualization; Data Curation; Formal Analysis; Visualization; Writing –
Original Draft; Writing – Review & Editing. AR: Funding Acquisition;
Supervision; Writing – Original Draft; Writing – Review & Editing.
References
Allison,
S.D., Martiny, J.B.H. 2008. Resistance, resilience,
and redundancy in microbial communities. Proceedings of the National Academy
of Sciences 105: 11512–11519. https://doi.org/10.1073/pnas.0801925105
Arce, M.I., Mendoza-Lera, C.,
Almagro, M., Catalán, N., Romaní, A.M., Martí, E., Gómez, R., et al. 2019. A conceptual framework for understanding the biogeochemistry of dry
riverbeds through the lens of soil science. Earth-Science Reviews 188:
441–453. https://doi.org/10.1016/j.earscirev.2018.12.001
Barnard, R.L., Osborne, C.A., Firestone, M.K. 2013. Responses of soil
bacterial and fungal communities to extreme desiccation and rewetting. The
ISME Journal 7: 2229–2241. https://doi.org/10.1038/ismej.2013.104
Barnard, R.L., Osborne, C.A., Firestone, M.K. 2014. Changing precipitation
pattern alters soil microbial community response to wet-up under a
Mediterranean-type climate. The ISME Journal 9: 946–957. https://doi.org/10.1038/ismej.2014.192
Barthès, A., Ten-Hage, L., Lamy, A., Rols, J.L., Leflaive, J. 2015.
Resilience of AggregatedMicrobial Communities Subjected to Drought—Small-Scale
Studies. Microbial Ecology 70: 9–20. https://doi.org/10.1007/s00248-014-0532-0
Battin, T.J., Besemer, K., Bengtsson, M.M., Romani, A.M., Packmann, A.I.
2016. The ecology and biogeochemistry of stream biofilms. Nature Reviews
Microbiology 14: 251–263. https://doi.org/10.1038/nrmicro.2016.15
Bonada, N., Resh, V.H.
2013. Mediterranean-climate streams and rivers: Geographically
separated but ecologically comparable freshwater systems. Hydrobiologia
719: 1–29. https://doi.org/10.1007/s10750-013-1634-2
Burns, R.G., DeForest, J.L., Marxsen, J., Sinsabaugh, R.L., Stromberger,
M.E., Wallenstein, M.D., Weintraub, M.N., et al. 2013. Soil enzymes in a
changing environment: Current knowledge and future directions. Soil Biology
and Biochemistry 58: 216–234. https://doi.org/10.1016/j.soilbio.2012.11.009
Casas-Ruiz, J.P., Tittel, J., von Schiller, D., Catalán, N., Obrador, B.,
Gómez-Gener, L., Zwirnmann, E., et al. 2016. Drought-induced discontinuities in
the source and degradation of dissolved organic matter in a Mediterranean
river. Biogeochemistry
127: 125–139. https://doi.org/10.1007/s10533-015-0173-5
Collins, R., Thyssen, N., Kristensen, P. 2009. Water
resources across Europe – Confronting water scarcity and drought. Publications
Office, European Environment Agency. https://data.europa.eu/doi/10.2800/16803
Costigan, K.H., Jaeger, K.L., Goss, C.W., Fritz, K.M., Goebel, P.C. 2016.
Understanding controls on flow permanence in intermittent rivers to aid
ecological research: integrating meteorology, geology and land cover. Ecohydrology 9: 1141–1153. https://doi.org/10.1002/eco.1712
Coulson, L.E., Weigelhofer, G., Gill, S., Hein, T.,
Griebler, C. Schelker, J. 2022. Small rain events during drought alter sediment dissolved organic
carbon leaching and respiration in intermittent stream sediments. Biogeochemistry
159: 159–178. https://doi.org/10.1007/s10533-022-00919-7
Datry, T., Bonada, N., Boulton, A.J. (eds.). 2017. Intermittent Rivers
and Ephemeral Streams - Ecology and Management. Elsevier.
Evans, S.E., Wallenstein,
M.D., Burke, I.C. 2014. Is bacterial
moisture niche a good predictor of shifts in community composition under
long-term drought. Ecology 95: 110–122. https://doi.org/10.1890/13-0500.1
Febria, C.M., Hosen, J.D., Crump, B.C., Palmer, M.A., Williams, D.D. 2015.
Microbial responses to changes in flow status in temporary headwater streams: a
cross-system comparison. Frontiers in Microbiology 6. https://doi.org/10.3389/fmicb.2015.00522
Findlay, S., 2010. Stream microbial ecology. Journal of the North
American Benthological Society: 29: 170–181. https://doi.org/10.1899/09-023.1
Freixa, A., Ejarque, E., Crognale, S., Amalfitano, S., Fazi, S., Butturini,
A., Romaní, A.M. 2016. Sediment microbial communities
rely on different dissolved organic matter sources along a Mediterranean river
continuum. Limnology and Oceanography: 61. https://doi.org/10.1002/lno.10308
Frossard, A., Gerull, L., Mutz, M., Gessner, M.O. 2012. Disconnect of
microbial structure and function: enzyme activities and bacterial communities
in nascent stream corridors. The ISME Journal 6: 680–691. https://doi.org/10.1038/ismej.2011.134
Gibbons, S.M., Jones, E., Bearquiver, A., Blackwolf, F., Roundstone, W.,
Scott, N., Hooker, J., et al. 2014. Human and environmental impacts on river
sediment microbial communities. PLoS One 9: 1–9. https://doi.org/10.1371/journal.pone.0097435
Gionchetta, G., Oliva,
F., Menéndez, M., Lopez Laseras, P., Romaní, A.M. 2019. Key
role of streambed moisture and flash storms for microbial resistance and
resilience to long-term drought. Freshwater Biology 64: 306–322. https://doi.org/10.1111/fwb.13218
Gionchetta, J., Artigas, J., Arias‐Real, R., Oliva, F., Romaní,
A.M. 2020. Multi‐model assessment of hydrological and environmental impacts on
streambed microbes in Mediterranean catchments. Environmental Microbiology
22: 2213-2229. https://doi.org/10.1111/1462-2920.14990
Gornall, J., Betts, R., Burke, E., Clark, R., Camp, J., Willett, K.,
Wiltshire, A. 2010. Implications of climate change for agricultural
productivity in the early twenty-first century. Philosophical Transactions
of the Royal Society B: Biological Sciences 365: 2973–2989. https://doi.org/10.1098/rstb.2010.0158
Görres, J.H., Savin, M.C., Neher, D.A., Weicht, T.R., Amador, J.A. 1999.
Grazing in a porous environment: 1. The effect of soil pore structure on C and
N mineralization. Plant and Soil 212: 75–83. https://doi.org/10.1023/A:1004694202862
Griffith, C.A., Shang, P., Lu, Y., Theuerkauf, E.J., Rodriguez, A.B.,
Findlay, R.H. 2019. Agricultural land use impacts microbial community structure
of streambed sediments. Aquatic Microbial Ecology 83: 225-236. https://doi.org/10.3354/ame01905
Harms, T.K.,
Grimm, N.B. 2012. Responses of trace gases to
hydrologic pulses in desert floodplains. Journal of Geophysical Research:
Biogeosciences 117: 1–14. https://doi.org/10.1029/2011JG001775
Jessup, C.M., Kassen, R., Forde, S.E., Kerr, B., Buckling, A., Rainey,
P.B., Bohannan, B.J.M. 2004. Big questions, small worlds: Microbial model
systems in ecology. Trends in Ecology and Evolution 19: 189–197. https://doi.org/10.1016/j.tree.2004.01.008
Jones, S.E.,
Lennon, J.T. 2010. Dormancy
contributes to the maintenance of microbial diversity. Proceedings of the
National Academy of Sciences 107: 5881–5886. https://doi.org/10.1073/pnas.0912765107
Larned, S.T., Datry, T., Arscott, D.B., Tockner, K. 2010. Emerging
concepts in temporary-river ecology. Freshwater Biology 55: 717–738. https://doi.org/10.1111/j.1365-2427.2009.02322.x
Lear, G., Bellamy, J., Case, B.S., Lee, J.E., Buckley, H.L. 2014.
Fine-scale spatial patterns in bacterial community composition and function
within freshwater ponds. The ISME Journal 8: 1715–1726. https://doi.org/10.1038/ismej.2014.21
Lesk, C., Rowhani, P., Ramankutty, N. 2016. Influence of extreme weather
disasters on global crop production. Nature 529: 84–87. https://doi.org/10.1038/nature16467
Logue, J.B., Stedmon, C.A., Kellerman, A.M., Nielsen, N.J., Andersson,
A.F., Laudon, H., Lindström, E.S., et al. 2016. Experimental insights into the
importance of aquatic bacterial community composition to the degradation of
dissolved organic matter. The ISME Journal 10: 533–545. https://doi.org/10.1038/ismej.2015.131
Louca, S., Polz, M.F., Mazel, F., Albright, M.B.N., Huber, J.A.,
O’Connor, M.I., Ackermann, M. et al. 2018. Function and functional redundancy
in microbial systems. Nature Ecology and Evolution 2: 936–943. https://doi.org/10.1038/s41559-018-0519-1
Manzoni, S., Schimel, J.P., Porporato, A. 2012. Responses
of soil microbial communities to water stress: results from a meta-analysis. Ecology
93: 930–938. https://doi.org/10.1890/11-0026.1
Marxsen, J., Zoppini, A., Wilczek, S. 2010. Microbial communities in
streambed sediments recovering from desiccation. FEMS Microbiology Ecology
71: 374–386. https://doi.org/10.1111/j.1574-6941.2009.00819.x
Meisner, A., Jacquiod, S., Snoek, B.L., Ten Hooven, F.C., van der Putten,
W.H. 2018. Drought legacy effects on the composition of soil fungal and
prokaryote communities. Frontiers in Microbiology 9: 1–12. https://doi.org/10.3389/fmicb.2018.00294
Monard, C., Gantner, S., Bertilsson, S., Hallin, S., Stenlid, J. 2016. Habitat
generalists and specialists in microbial communities across a
terrestrial-freshwater gradient. Scientific Reports 6: 1–10. https://doi.org/10.1038/srep37719
Morandi, B., Piégay, H., Lamouroux, N., Vaudor, L. 2014. How is success or failure in river restoration projects evaluated?
Feedback from French restoration projects. Journal of Environmental
Management 137: 178–188. https://doi.org/10.1016/j.jenvman.2014.02.010
Mori, N., Simčič, T., Brancelj, A., Robinson, C.T., Doering, M. 2017. Spatiotemporal heterogeneity of actual and potential respiration in
two contrasting floodplains. Hydrological Processes 31: 2622–2636. https://doi.org/10.1002/hyp.11211
Naylor,
D., Coleman-Derr, D. 2018. Drought
Stress and Root-Associated Bacterial Communities. Frontiers in Plant Science
8: 1–16. https://doi.org/10.3389/fpls.2017.02223
Prudhomme, C., Giuntoli, I., Robinson, E.L., Clark, D.B., Arnell, N.W.,
Dankers, R., Fekete, B.M., et al. 2014. Hydrological droughts in the 21st
century, hotspots and uncertainties from a global multimodel ensemble
experiment. Proceedings of the National Academy of Sciences 111:
3262–3267. https://doi.org/10.1073/pnas.1222473110
Romaní, A.M., Amalfitano, S., Artigas, J., Fazi, S., Sabater, S., Timoner,
X., Ylla, I., Zoppini, A. 2013. Microbial biofilm
structure and organic matter use in mediterranean streams. Hydrobiologia
719: 43–58. https://doi.org/10.1007/s10750-012-1302-y
Romaní, A.M., Chauvet, E., Febria, C., Mora-Gómez, J., Risse-Buhl, U.,
Timoner, X., Weitere, M., et al. 2017. The biota of intermittent rivers and
ephemeral streams: prokaryotes, fungi and protozoans. In: Datry, T., Bonada,
N., Boulton, A.J. (eds.), Intermittent Rivers and Ephemeral Streams -
Ecology and Management, pp. 161-188. Elsevier.
Sabater, S., Timoner, X., Borrego, C., Acuña, V. 2016. Stream biofilm
responses to flow intermittency: from cells to ecosystems. Frontiers in
Environmental Science 4: 1–10. https://doi.org/10.3389/fenvs.2016.00014
Schimel, J., Balser, T.C., Wallenstein, M. 2007. Microbial stress-response
physiology and its implications for ecosystem function. Ecology 88:
1386–1394. https://doi.org/10.1890/06-0219
Schimel, J.P., Boot, C., Holden, P.A., Roux-Michollet, D., Parker, S.,
Schaeffer, S., Treseder, K.K. 2010. Enzyme activity and adaptation in dry soil.
19th World Congress of Soil Science, Soil Solutions for a Changing World, 1
– 6 August 2010, Brisbane, Australia, pp. 17–20.
Shumilova, O., Zak, D.,
Datry, T., von Schiller, D., Corti, R., Foulquier, A., Obrador, B., et al.
2019. Simulating rewetting events in intermittent rivers and
ephemeral streams: A global analysis of leached nutrients and organic matter. Global
Change Biology 25(5): 1591–1611. https://doi.org/10.1111/gcb.14537
Sinsabaugh, R.L. 2010. Phenol oxidase, peroxidase and organic matter dynamics
of soil. Soil Biology and Biochemistry 42: 391-404.
https://doi.org/10.1016/j.soilbio.2009.10.014
Stefan, G., Cornelia, B., Römbke, J., Bonkowski, M. 2014. Soil water
availability strongly alters the community composition of soil protists. Pedobiologia 57: 205–213. https://doi.org/10.1016/j.pedobi.2014.10.001
Steward, A.L., Von Schiller, D., Tockner, K., Marshall, J.C., Bunn, S.E.
2012. When the river runs dry: Human and ecological
values of dry riverbeds. Frontiers in Ecology and the Environment 10:
202–209. https://doi.org/10.1890/110136
Timoner, X., Acuña, V., Von Schiller, D., Sabater, S. 2012. Functional
responses of stream biofilms to flow cessation, desiccation and rewetting. Freshwater
Biology 57: 1565–1578. https://doi.org/10.1111/j.1365-2427.2012.02818.x
Vadher, A.N., Leigh, C., Millett, J., Stubbington, R., Wood, P.J. 2017.
Vertical movements through subsurface stream sediments by benthic
macroinvertebrates during experimental drying are influenced by sediment
characteristics and species traits. Freshwater Biology 62: 1730–1740. https://doi.org/10.1111/fwb.12983
Wagner, K., Bengtsson, M.M., Besemer, K., Sieczko, A., Burns, N.R.,
Herberg, E.R., Battin, T.J. 2014. Functional and Structural Responses of
Hyporheic Biofilms to Varying Sources of Dissolved Organic Matter. Applied
and Environmental Microbiology 80: 6004–6012. https://doi.org/10.1128/AEM.01128-14
Weitere, M., Erken, M., Majdi, N., Arndt, H., Norf, H., Reinshagen, M.,
Traunspurger, W., et al. 2018, The food web perspective on aquatic biofilms. Ecological
Monographs 88: 543-559. https://doi.org/10.1002/ecm.1315
Wilhite, D.A. 2000. Drought as a Natural Hazard: Concepts and Definitions.
In: Wilhite, D.A. (ed.) Drought: A Global Assessment, Vol.I, pp. 3–18.
Drought Mitigation Center Faculty Publications, London.
Ylla, I., Sanpera-Calbet, I.,
Vázquez, E., Romaní, A.M., Muñoz, I., Butturini, A., Sabater, S. 2010. Organic matter availability during pre- and post-drought periods in
a Mediterranean stream. Hydrobiologia 657: 217–232. https://doi.org/10.1007/s10750-010-0193-z
Yuste, J.C., Peñuelas, J.,
Estiarte, M., Garcia-Mas, J., Mattana, S., Ogaya, R., Pujol, M., et al. 2011. Drought-resistant fungi control soil organic matter decomposition
and its response to temperature. Global Change Biology 17: 1475–1486. https://doi.org/10.1111/j.1365-2486.2010.02300.x
Zeglin, L.H. 2015. Stream microbial diversity in response to environmental
changes: review and synthesis of existing research. Frontiers
in Microbiology 6: 454. https://doi.org/10.3389/fmicb.2015.00454
Zoppini, A., Ademollo, N., Amalfitano, S., Casella, P., Patrolecco, L.,
Polesello, S. 2014. Organic priority substances and
microbial processes in river sediments subject to contrasting hydrological
conditions. Science of the Total Environment 484: 74–83. https://doi.org/10.1016/j.scitotenv.2014.03.019
![]() ,
Anna Maria Romaní2
,
Anna Maria Romaní2 ![]()



